The RINVOQ study program1
RINVOQ demonstrated consistent clinical remission rates across patient populations studied1
*RINVOQ is not indicated for MTX-naive patients.
†Primary endpoint: clinical remission (DAS28 [CRP]<2.6) compared to placebo.
bDMARD: biological disease-modifying antirheumatic drug; csDMARD-IR: inadequate responder to conventional synthetic DMARDs; DAS28 (CRP): disease activity score with 28 joint count (C-reactive protein); DMARD: disease-modifying antirheumatic drug; MTX: methotrexate.
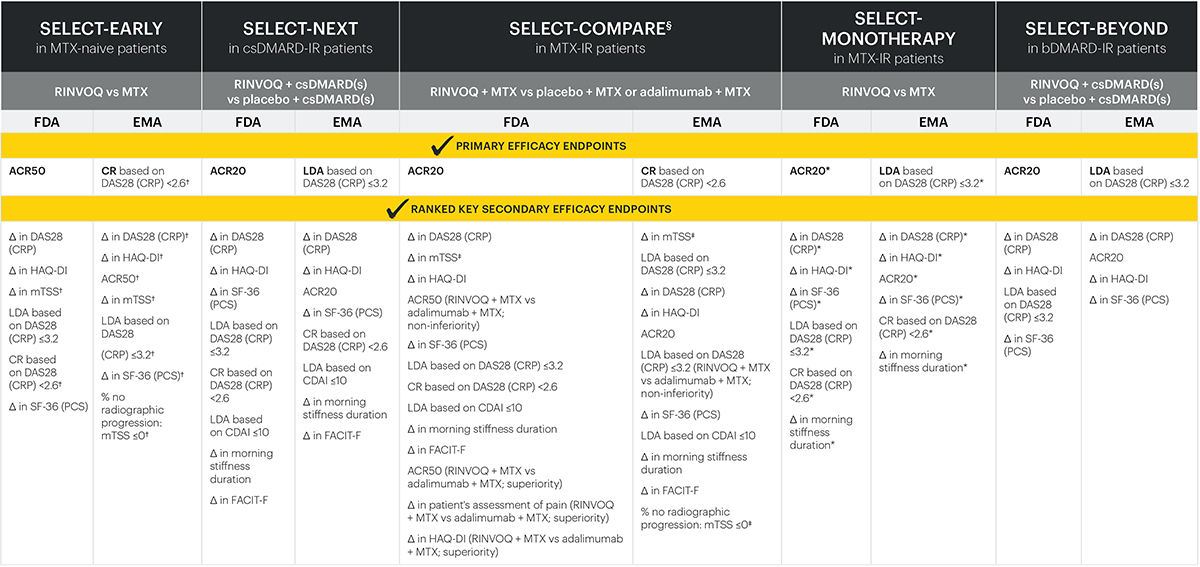
RINVOQ met every primary and secondary endpoint in its Phase 3 registrational studies1-6
Endpoints across all studies
RINVOQ is not indicated for MTX-naive patients. All primary and ranked key secondary endpoints were multiplicity-controlled and met statistical significance denoted by a P-value ≤0.05. All endpoints were assessed at Week 12, if not indicated otherwise:
*Assessment at Week 14.
†Assessment at Week 24.
‡Assessment at Week 26.
§In SELECT-COMPARE, all comparisons were for RINVOQ + MTX vs placebo + MTX, unless indicated otherwise.
ACR20/50: American College of Rheumatology 20/50 response; CDAI: Clinical Disease Activity Index; CR: clinical remission; CRP: C-reactive protein; csDMARD: conventional synthetic disease-modifying antirheumatic drug; DAS28 (CRP): disease activity score with 28-joint count (C-reactive protein); EMA: European Medicines Agency; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; FDA: Food and Drug Administration; HAQ-DI: Health Assessment Questionnaire-Disability Index; LDA: low disease activity; mTSS: modified total Sharp score; MTX: methotrexate; PCS: physical component summary; SF-36: Short Form-36.
RINVOQ safety profile in RA accross multiple patient populations in six Phase 3 studies7
including data from SELECT-CHOICE*; integrated safety analysis of RINVOQ up to 4,5 years max exposure.
*SELECT-CHOICE was not included as part of RINVOQ's registrational clinical study program.
†Patients who switched to RINVOQ from placebo (PBO), ADA, MTX, or abatacept (ABA) were included in the RINVOQ analysis set from the start of RINVOQ treatment; those who switched from RINVOQ to ADA were included in the ADA analysis set from the start of ADA treatment.
‡Excluding tuberculosis, oral candidiasis, and herpes zoster. The most common opportunistic infections reported with UPA were esophageal candidiasis and oral fungal infection.
§Cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
IIDeep vein thrombosis and pulmonary embolism.
Only data from treatment groups with long-term exposures were analyzed, therefore, data for PBO or ABA (which were only available for the short-term, double-blind controlled periods) were not included. SELECT-CHOICE data was included in the analysis set, although it is not part of the SELECT registrational clinical trial program.
MTX monotherapy data were censored at the time of rescue to combination therapy (i.e., addition of RINVOQ).
Treatment-emergent adverse events (AEs) were defined as any AE with an onset date on or after the first dose of study drug and <30 days (70 days for ADA) after the last dose of study drug in the event of premature study drug discontinuation AEs were defined using standardized Medical Dictionary for Regulatory Activities (MedDRA) query or company MedDRA query search criteria.
All deaths and cardiovascular (CV) events, including venous thromboembolism (VTE), across the clinical program were adjudicated by a blinded external CV adjudication committee.
Exposure-adjusted event rates (EAERs; total number of events [including incident and recurrent events in the same patient] adjusted for RINVOQ total exposure, expressed as events/100 patient-years [E/100 PY]), were reported for AEs and AEs of special interest for each of the treatment groups.
MTX: methotrexate; PYs: patient years.
Minimiinformation
RINVOQ® (upadacitinib), depottablett 15 mg, 30 mg, 45 mg (F), Rx, ATC-kod L04AF03 JAK-hämmare. Indikationer: måttlig till svår aktiv reumatoid artrit hos vuxna med otillräckligt behandlingssvar på eller intolerans mot ett eller flera DMARDs i monoterapi eller i kombination med metotrexat. Aktiv psoriasisartrit hos vuxna med otillräckligt behandlingssvar på eller intolerans mot ett eller flera DMARDs, i monoterapi eller i kombination med metotrexat. Axial spondylartrit: – Aktiv icke-radiografisk axial spondylartrit hos vuxna med objektiva tecken på inflammation som anges av förhöjda nivåer av CRP och/eller MRI, som har otillräckligt behandlingssvar på NSAID. – Aktiv ankyloserande spondylit (radiografisk axial spondylartrit) hos vuxna med otillräckligt behandlingssvar på konventionell behandling. Måttlig till svår atopisk dermatit hos vuxna och ungdomar 12 år och äldre vilka är aktuella för systemisk behandling. Måttlig till svår aktiv ulcerös kolit eller Crohns sjukdom hos vuxna med otillräckligt behandlingssvar, förlorat behandlingssvar eller som varit intoleranta mot konventionell behandling eller biologiska läkemedel. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne. Aktiv tuberkulos (TB) eller aktiv allvarlig infektion. Gravt nedsatt leverfunktion. Graviditet. Effekter på förmågan att framföra fordon: RINVOQ kan ha en liten effekt på förmågan att framföra fordon och använda maskiner eftersom yrsel och vertigo kan inträffa under behandling. Varningar och försiktighet: RINVOQ ska endast användas om inga lämpliga behandlingsalternativ är tillgängliga för patienter: som är 65 år eller äldre; med en anamnes på aterosklerotisk hjärt-kärlsjukdom eller andra kardiovaskulära riskfaktorer (t.ex. nuvarande eller tidigare långtidsrökare); med riskfaktorer för malignitet (t.ex. nuvarande eller tidigare malignitet). RINVOQ ska inte påbörjas hos patienter med aktiva, allvarliga infektioner, inkl. lokala infektioner och TB. Virusreaktivering, inkl. fall av reaktivering av herpesvirus (t.ex. herpes zoster), har rapporterats i kliniska studier. Påbörja inte eller avbryt tillfälligt behandling om onormala lab-värden som anemi, neutropeni, lymfopeni och levertransaminaser påträffas. RINVOQ associerades med ökade lipidparametrar i kliniska studier. Divertikulit och gastrointestinal perforation har rapporteras i kliniska prövningar och från klinisk erfarenhet. Patienter med aktiv Crohns sjukdom löper ökad risk att utveckla tarmperforation. VTE har rapporterats hos patienter på RINVOQ. Hos patienter med kända VTE-riskfaktorer ska RINVOQ användas med försiktighet. Allvarliga överkänslighetsreaktioner har rapporterats med RINVOQ. Fertilitet, graviditet och amning: Fertila kvinnor ska rådas använda effektiv preventivmetod under behandling och i minst 4 veckor efter avslutad behandling. RINVOQ är kontraindicerat under graviditet och ska inte användas under amning. För ytterligare information samt priser se www.fass.se. För information: kontakta AbbVie AB, 08 684 44 600. Datum för översyn av produktresumén: 24 oktober 2024. Begränsning av läkemedelsförmån: RINVOQ subventioneras 1) när behandling med TNF-hämmare gett otillräcklig effekt eller inte är lämplig 2) för patienter med atopisk dermatit när konventionell topikal eller systemisk behandling gett otillräcklig effekt eller inte är lämplig.
SE-RNQ-220026 v 9.0 Senast uppdaterad 2024-11-20
Referenser
- RINVOQ produktresumé
- Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase 3, double-blind, randomized controlled trial [published online July 9, 2019]. Arthritis Rheumatol. doi:10.1002/art.41032
- Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303-2311. doi:10.1016/S0140-6736(19)30419-2
- van Vollenhoven R, Takeuchi T, Pangan AL, et al. A phase 3 randomized, controlled trial comparing upadacitinib monotherapy to MTX monotherapy in MTX-naive patients with active rheumatoid arthritis. Presented at: American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Meeting; October 19–24, 2018; Chicago, IL.
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503-2512. doi:10.1016/S0140-6736(18)31115-2
- Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513-2524. doi:10.1016/S0140- 6736(18)31116-4
- Cohen B, van Vollenhoven, Curtis JR, et al. POS0220. Integrated Safety Profile of Upadacitinib With up to 4.5 Years of Exposure in Patients With Rheumatoid Arthritis. Poster presented at EULAR 2021
SE-RNQ-220007 v 2.0 Senast uppdaterad 2022-10-12