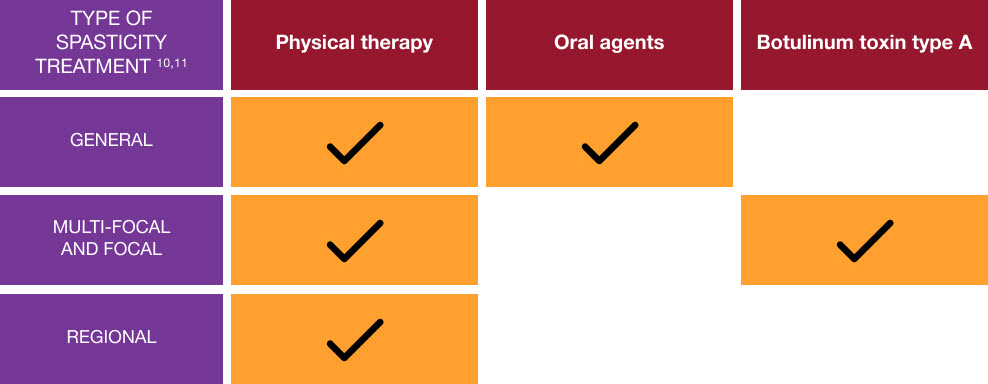
Treatment options for spasticity in adults
Diagnosis and treatment of PSS during the first 3 months post-stroke may benefit patients in their aim for a full recovery6,7
Combining physical therapy and BOTOX® may help patients reach their goals.8–10
Adapted from Royal College of Physicians, 201811 and Bhakta BB, 200012
BOTOX® should only be used for the treatment of focal spasticity in adult post-stroke patients if muscle tone reduction is expected to result in improved function (e.g. improvements in gait), or improved symptoms (e.g. reduction in muscle spasms or pain), and/or to facilitate care.5
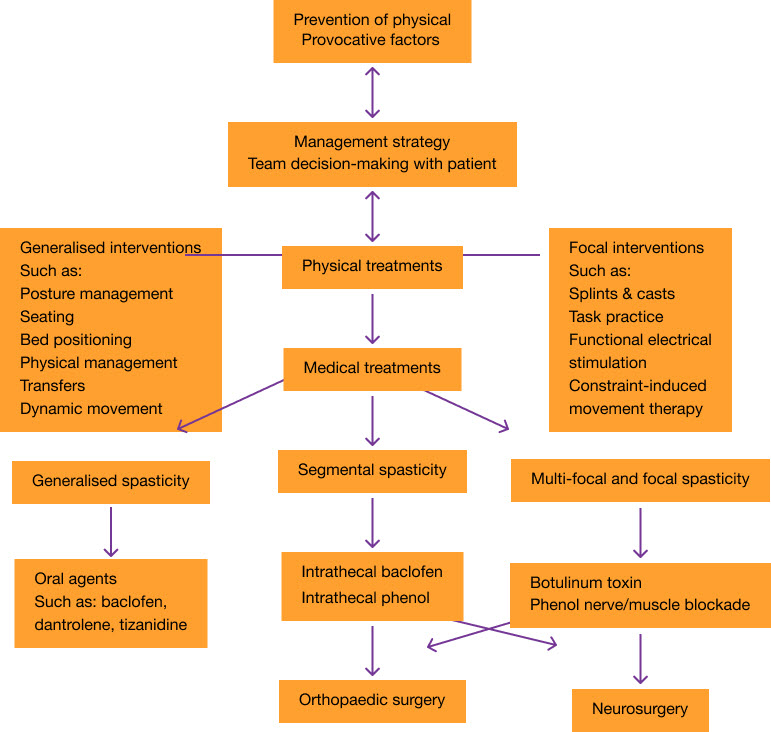
Management strategy for adults with spasticity11
Adapted from Royal College of Physicians 201811
PSS: post-stroke spasticity.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250072.