What is post-stroke spasticity (PSS)?
What is spasticity?
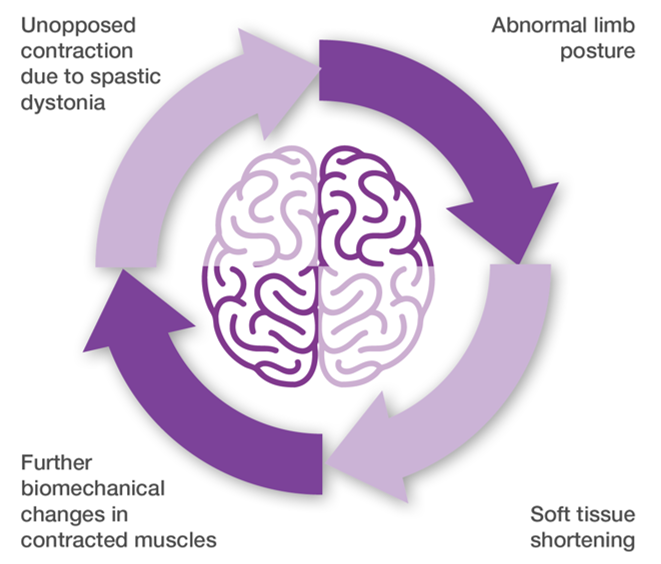
- Spasticity is a common consequence of neurological disorders, such as stroke,6 and presents as intermittent or sustained involuntary activation of muscles7
- Spasticity primarily affects the shoulder, elbow, forearm, wrist, fingers/hand, thumb, ankle and foot.5
- If left untreated, spasticity can cause shortening of muscles and tendons, and a vicious cycle can develop8
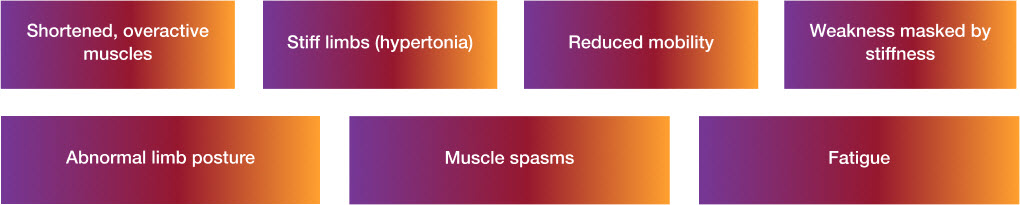
Adapted from Royal College of Physicians. Spasticity in adults: management using botulinum toxin, 20188
Features of spasticity8
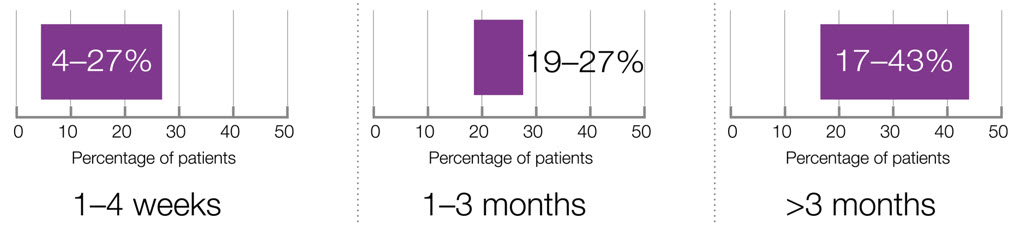
Prevalence of PSS increases with post-stroke survival time9
TIME POST-STROKE:
Adapted from Wissel J et al, 2013.9
In a prospective cohort study of patients after a first ischaemic stroke, when 211 patients were assessed after 6 months, 43% had developed spasticity and 15.6% had developed a more severe degree of spasticity (MAS ≥3).10
A timely diagnosis may avoid worsening of untreated spasticity8
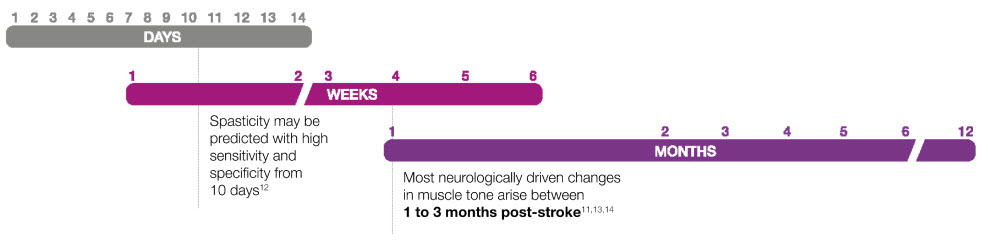
Post-stroke timeline
Neural changes may drive the onset of post-stroke spasticity9,11
If patients are not treated in a timely manner their spasticity may become more disabling over time, causing pain, deformity and contracture.15,16
A switch to intrinsic muscle remodelling between 3 and 18 months may lead to chronic severe spasticity, preventing a full recovery.9, 11, 15, 16
47% of patients do not receive botulinum toxin within a year of developing spasticity (N=132).17
Rehabilitation therapies applied within the first 3 months post-stroke are likely to deliver the most benefit.18
MAS, Modified Ashworth Scale; PSS: post-stroke spasticity.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250069.