Guidelines recommend BOTOX® as a treatment option in the management of post-stroke spasticity (PSS)6
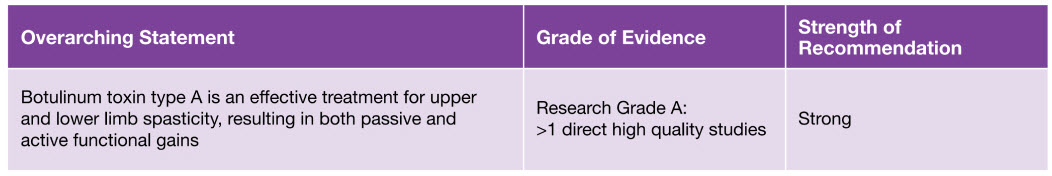
Botulinum toxin type A is a recommended option in the Royal College of Physicians National Guidelines (2018) - Spasticity in adults: management using botulinum toxin6
Royal College of Physicians – Spasticity in adults: Management using botulinum toxin (National Guidelines 2018)6
Early intervention: When combined with rehabilitation, botulinum toxin has been shown to provide a sustained reduction in post-stroke upper-limb spasticity as early as 2–12 weeks post-stroke (Rosales, Kong et al 2012)
Multidisciplinary approach: Botulinum toxin should be used as part of a coordinated multidisciplinary approach, involving physical management and therapy
Role for physiotherapists and nurses: In accordance with UK statutes, appropriately experienced and qualified physiotherapists and nurses may be trained to prescribe and inject botulinum toxin; this may be highly cost-efficient
Secondary benefits: A successful treatment package can also prevent secondary complications such as impaired movement and difficulty maintaining hygiene and self-care
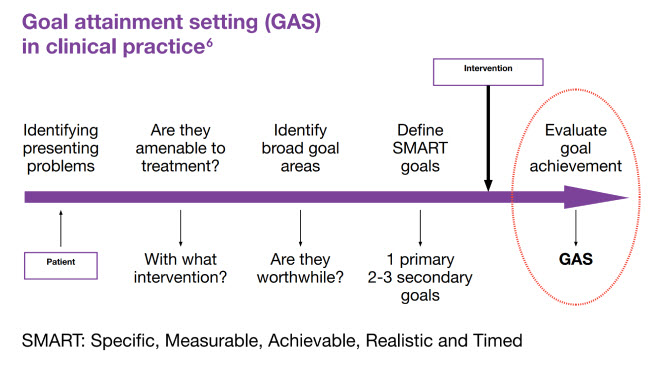
Core principles for intervention6
The principles for successful botulinum toxin intervention are:
 Appropriate patient selection (e.g. dynamic spastic component as opposed to contracture)
Appropriate patient selection (e.g. dynamic spastic component as opposed to contracture)
 Clear, realistic and worthwhile goals for treatment agreed with patients and families (e.g. pain relief; reduction of involuntary movements; mobility)
Clear, realistic and worthwhile goals for treatment agreed with patients and families (e.g. pain relief; reduction of involuntary movements; mobility)
 Establishment of the immediate and ongoing treatment programme
Establishment of the immediate and ongoing treatment programme
Adapted from Royal College of Physicians 20186
GAS: goal attainment setting; PSS: post-stroke spasticity; SMART: Specific, Measurable, Achievable, Realistic and Timed.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250067.