Administering BOTOX® for your post-stroke spasticity (PSS) patients
BOTOX® has proven long-term evidence and experience in PSS in the upper and lower limb6, 7
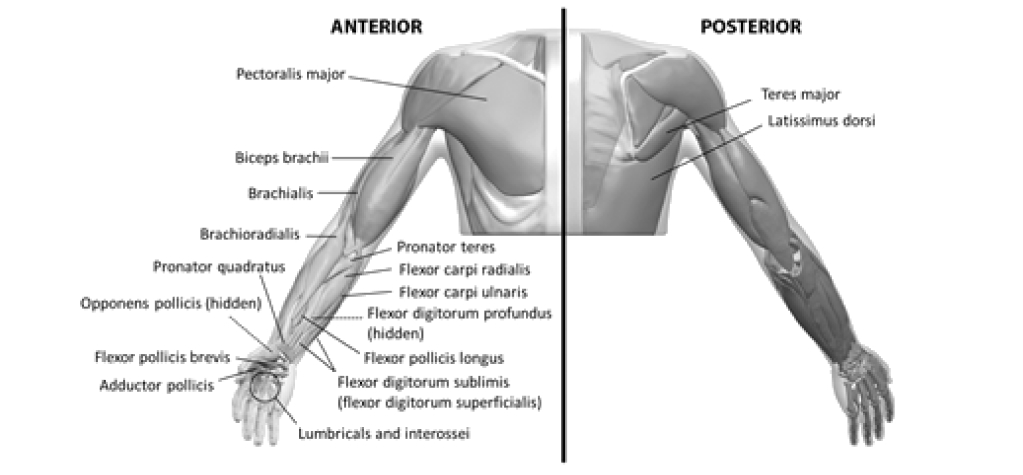
- Localisation of the involved muscles with techniques such as needle electromyographic guidance, nerve stimulation, or ultrasound is recommended5
- Multiple injection sites may allow BOTOX® to have more uniform contact with the innervation areas of the muscle and are especially useful in larger muscles5
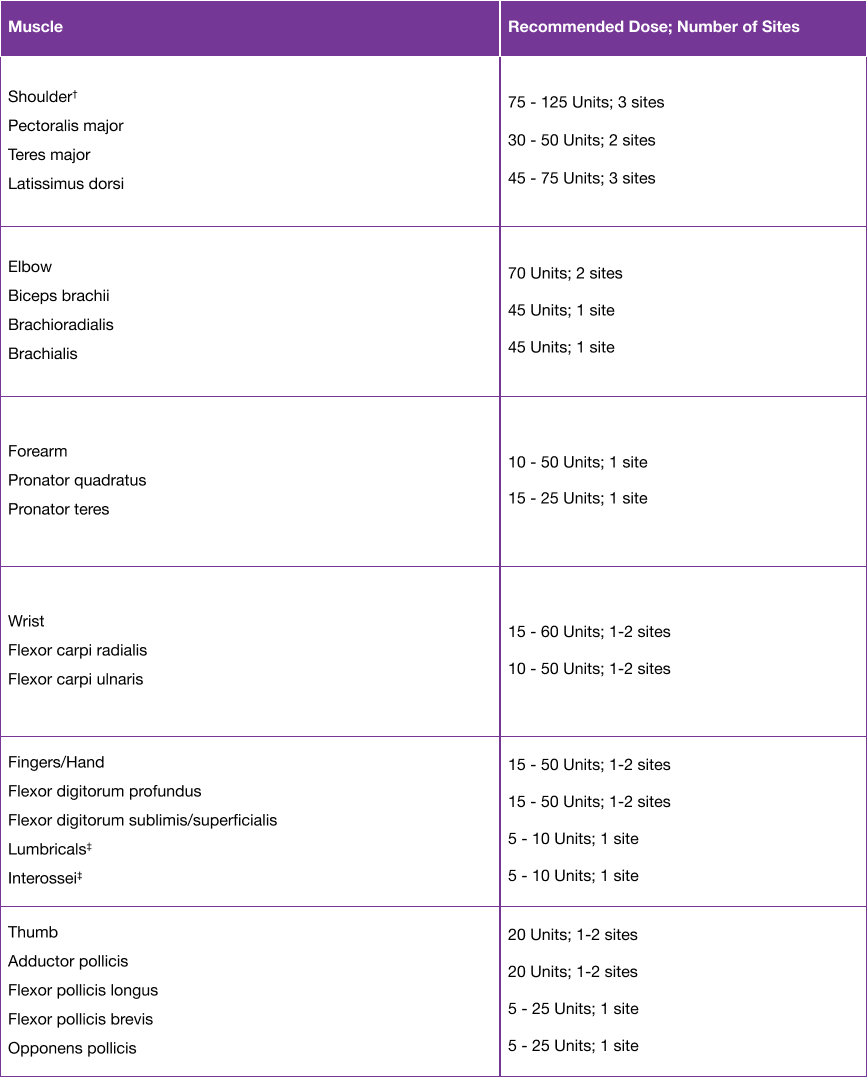
- The recommended dose for treating adult upper limb spasticity is up to 400 Units divided among the affected muscles5
- The exact dosage and number of injection sites may be tailored to the individual based on the size, number and location of muscles involved, the severity of spasticity, the presence of local muscle weakness, and the patient response to previous treatment5
- Localisation of the involved muscles with techniques such as electromyographic guidance, nerve stimulation or ultrasound is recommended5
Please refer to the dilution table in the SmPC. For the patient preparation and post-injection monitoring, please see Section 4.4 of the SmPC.5
†When injecting the shoulder muscles in combination, the recommended maximum dose is 250 U.5
‡When injecting both lumbricals and/or interossei, the recommended maximum dose is 50 U per hand.5
- The recommended dose for treating adult upper limb spasticity is up to 400 Units divided among the affected muscles5
- The exact dosage and number of injection sites may be tailored to the individual based on the size, number and location of muscles involved, the severity of spasticity, the presence of local muscle weakness, and the patient response to previous treatment5
- Localisation of the involved muscles with techniques such as electromyographic guidance, nerve stimulation or ultrasound is recommended5
Please refer to the dilution table in the SmPC. For the patient preparation and post-injection monitoring, please see Section 4.4 of the SmPC.5
†When injecting the shoulder muscles in combination, the recommended maximum dose is 250 U.5
‡When injecting both lumbricals and/or interossei, the recommended maximum dose is 50 U per hand.5
MAS: Modified Ashworth Scale; PSS: post-stroke spasticity; SmPC: summary of product characteristics.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250068.