Resources
For healthcare professionals
Post-Stroke Spasticity Risk Classification System
This risk classification system has been developed by a panel of international experts in PSS and AbbVie. This tool is designed to support healthcare professionals when evaluating patients who have had a stroke, ideally within the first 12 weeks post-stroke and recommended to be used at regular follow-up visits too.
This screening tool provides information on the criteria that determine whether post-stroke patients receive an urgent referral, routine referral or periodic monitoring and includes the next steps that should be considered.
To receive a copy of this risk classification system, please submit your details on the Contact Us page and tick Post-Stroke Spasticity Risk Classification System.
Post-stroke Spasticity Early Identification Guide
This guide is for healthcare professionals who manage stroke patients who may be at risk of developing PSS or who have PSS.
This guide contains information about the prevalence of PSS, the impact of PSS on patients’ lives, the risk factors that may contribute to the development of PSS and most importantly highlighting the importance of early identification of these patients to avoid worsening of untreated spasticity as supported by the Royal College of Physicians National Guidelines 2018.
To receive a copy of this guide, please submit your details on the Contact Us page and tick Post-stroke Spasticity Early Identification Guide.
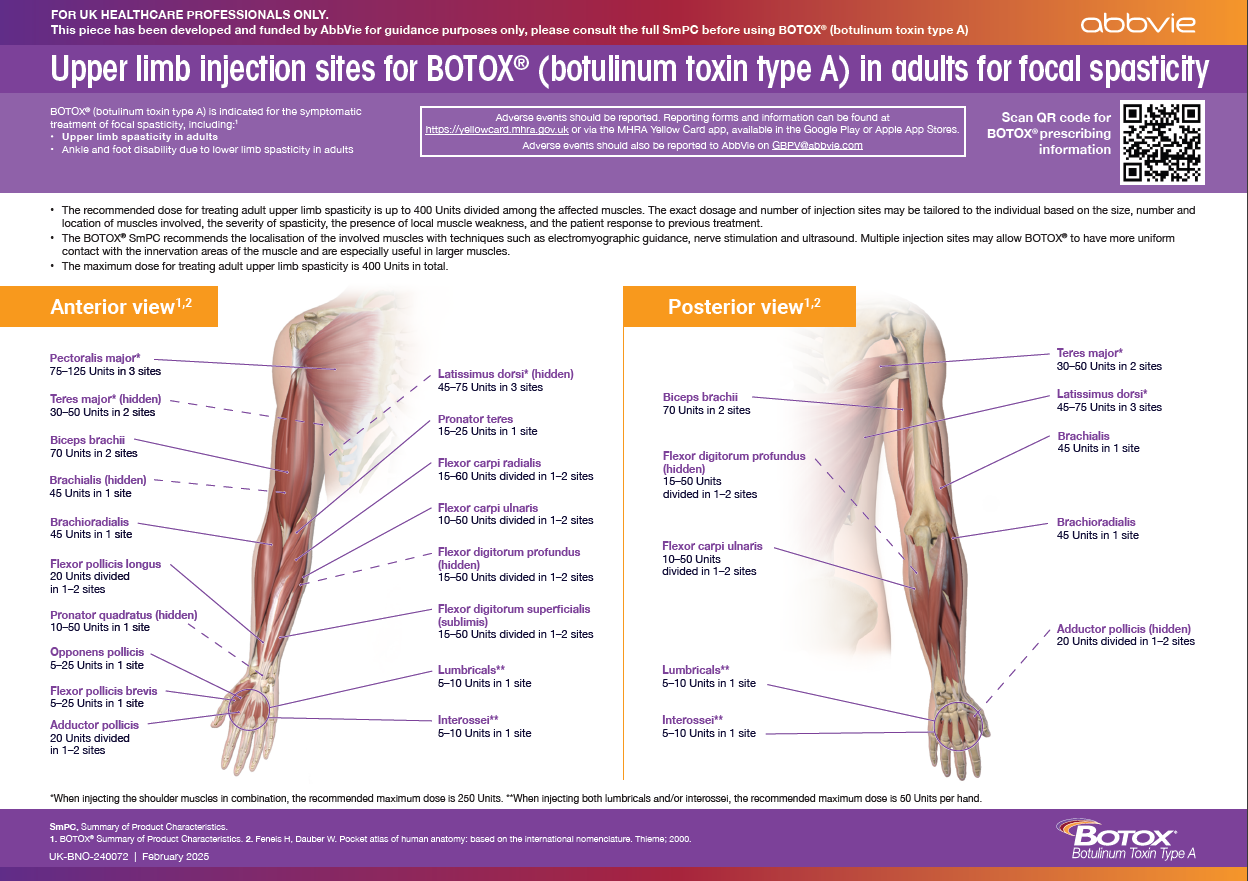
Upper limb injection sites for BOTOX® in adults for focal spasticity
This material is for healthcare professionals who are injecting BOTOX® to provide guidance regarding which muscles can be injected for upper limb spasticity.
To receive a copy of this guide, please submit your details on the Contact Us page and tick Upper limb injection sites for BOTOX® in adults for focal spasticity.
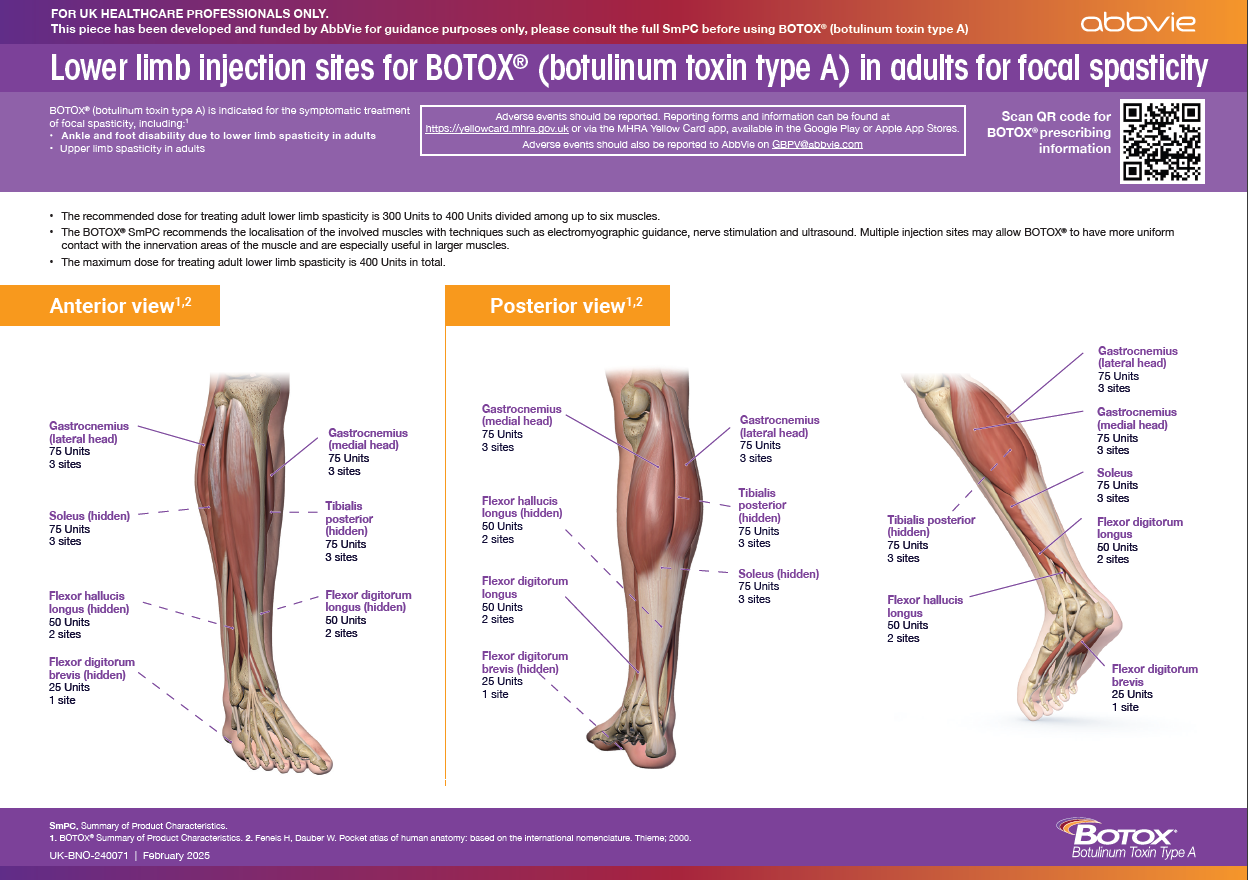
Lower limb injection sites for BOTOX® in adults for focal spasticity
This material is for healthcare professionals who are injecting BOTOX® to provide guidance regarding which muscles can be injected for lower limb spasticity.
To receive a copy of this guide, please submit your details on the Contact Us page and tick Lower limb injection sites for BOTOX® in adults for focal spasticity.
BOTOX® dosage and administration guide.
This guide provides a summary of the dosage and administration for BOTOX® and offers additional useful information on contraindications, injection sites, and recommended treatment intervals.
To receive a copy of this guide, please submit your details on the Contact Us page and tick BOTOX® dosage and administration guide.
Educational resources for healthcare professionals
Focal Spasticity E-learning Module 1: Introduction to Focal Spasticity.
This AbbVie Pro module is designed for healthcare professionals and will cover how spasticity is defined, the pathophysiology of focal spasticity, its features and explain when spasticity may develop after a stroke.
To access this module please click here
Focal Spasticity E-learning Module 2: Impact of Focal Spasticity
This AbbVie Pro module is designed for healthcare professionals and will outline the impact of focal spasticity on patients’ lives and explain the wider impact of focal spasticity (specifically post-stroke spasticity) on patients and their caregivers.
To access this module please click here
Focal Spasticity E-learning Module 3: Diagnosis and Management of Focal Spasticity
This AbbVie Pro module is designed for healthcare professionals and will highlight the importance of early identification of focal spasticity, explain the potential risk factors for its development, detail the methods used for assessing cases, outline its management approaches and provide information on setting goals for patients with this condition.
To access this module please click here
For your patients
A guide for patients being treated with BOTOX® (botulinum toxin type A) for focal spasticity
To support your patients with focal spasticity that have been prescribed BOTOX®, this guide provides information on how BOTOX® works, what patients can expect during and after treatment and any potential side-effects of BOTOX® treatment.
The information in this guide does not replace the information in the Patient Information Leaflet that is included with the prescribed medication, but is supplementary.
To receive a copy of this guide, please submit your details on the Contact Us page and tick: A guide for patients being treated with BOTOX® (botulinum toxin type A) for focal spasticity.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250036.