Safety and tolerability experience from >30 years of use in a range of conditions5-7
BOTOX® is generally well tolerated in the treatment of post-stroke spasticity (PSS)5
In a meta-analysis of data from 37 randomised controlled trials across all indications (BOTOX® n=1,447):6
- Adverse events (AEs) associated with BOTOX® were generally mild to moderate in severity
- Results were consistent over long-term clinical use
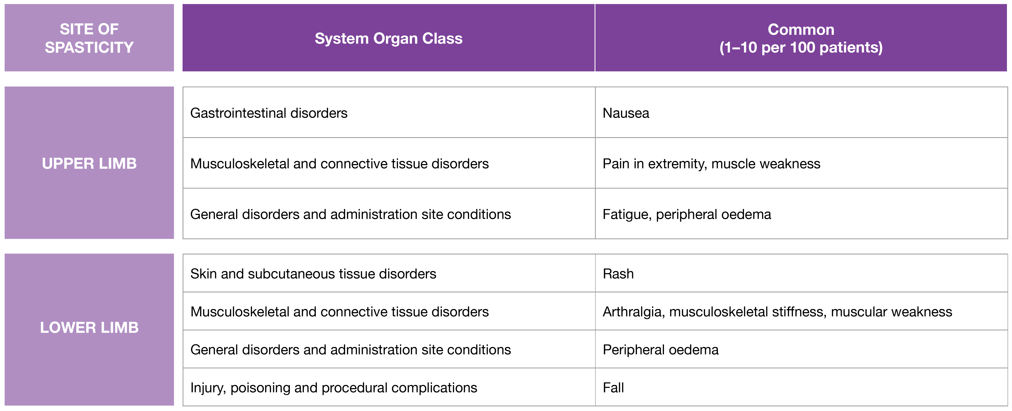
Adapted from the BOTOX® Summary of Product Characteristics.5
Please refer to the BOTOX® Summary of Product Characteristics for the full list of adverse events.
PSS: post-stroke spasticity.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BTX-250066.