This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
Neurogenic detrusor overactivity (NDO) can be debilitating and negatively affect patients’ quality of life6
Spinal lesions caused by trauma, such as spinal cord injury (SCI), or by a progressive neurological condition, such as multiple sclerosis (MS) can lead to NDO7,8
The consequences of untreated NDO can result in hospitalisation with life-threatening conditions such as:6,9,10
Urinary dysfunction is very common in patients with MS and SCI
- Up to 80% of patients with MS report some form of urinary incontinence11
- 81% of patients with SCI report some degree of bladder dysfunction within 1 year of injury12
Bladder symptoms are often inadequately managed
 Patients with MS have bladder symptoms that are often not adequately managed13
Patients with MS have bladder symptoms that are often not adequately managed13
 Approximately 51% of patients with moderate to severe LUTS are on anticholinergic therapy14
Approximately 51% of patients with moderate to severe LUTS are on anticholinergic therapy14
• <14% of patients started on oxybutynin and tolterodine (two of the most common oral OAB medications) continued treatment
for 1 year15
• Median of 31 days until discontinuation15
 Poor oral therapy adherence may be linked to:13
Poor oral therapy adherence may be linked to:13
• UTI, retention and obstruction
• Systemic complications
• Increased hospitalisation rates
 Urologists/urogynaecologists should work closely with neurologists in order to optimally manage NDO
Urologists/urogynaecologists should work closely with neurologists in order to optimally manage NDO
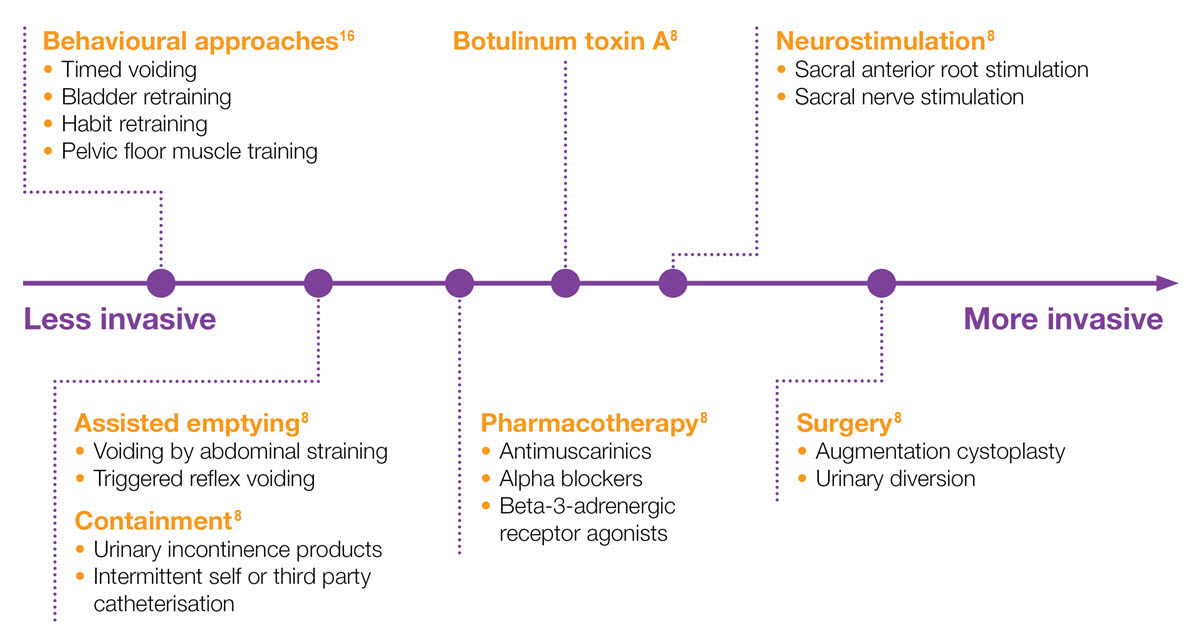
Spectrum of treatments for NDO8,16
Adapted from EAU Guidelines 2020 and NICE Guidelines 20128,16
NB: not all treatments mentioned here are licensed for NDO in the UK.
LUTS: lower urinary tract symptoms; MS: multiple sclerosis; NDO, neurogenic detrusor overactivity; OAB: overactive bladder; SCI: spinal cord injury; UTI: urinary tract infection.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250050.