This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
Considerations and guidance for using BOTOX® as a treatment option for managing neurogenic detrusor overactivity (NDO)
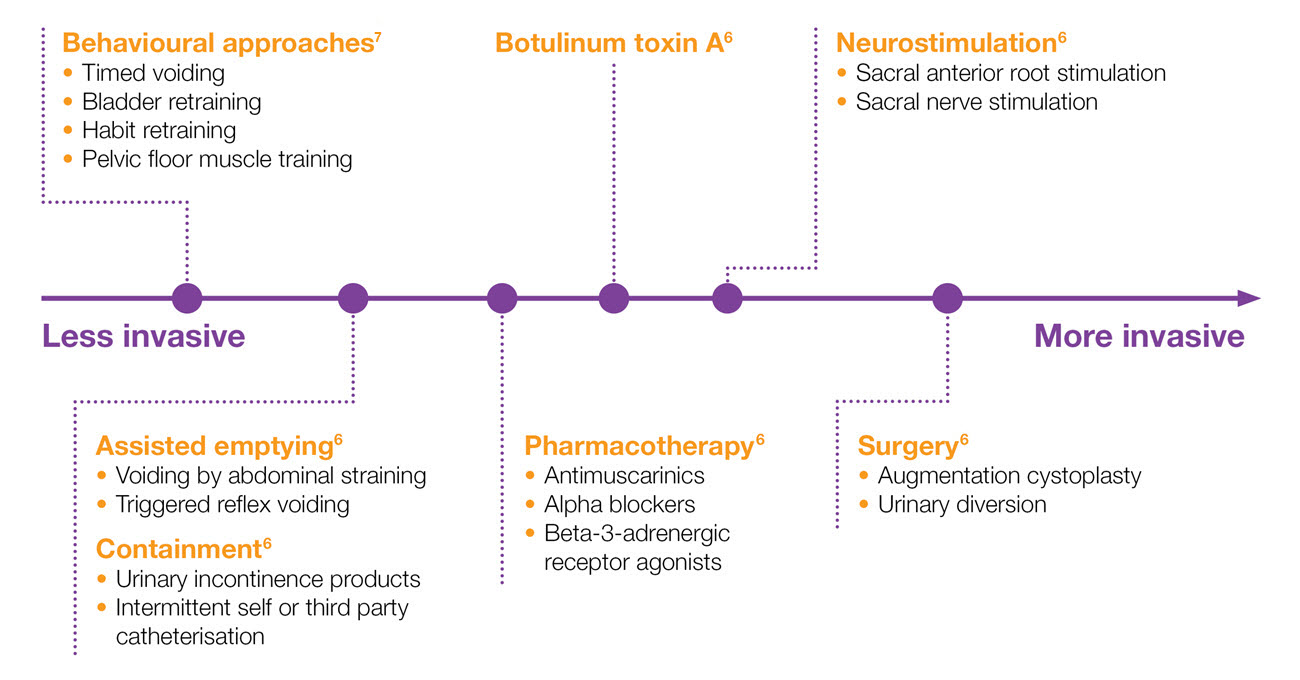
Spectrum of treatments for NDO6,7
Adapted from EAU Guidelines 2022 and NICE Guidelines 20126,7
Botulinum toxin injection is a recommended option in the ICI and NICE guidelines7,8
BoNT/A: botulinum toxin type-A; ICI: International Consultation on Incontinence; NDO: neurogenic detrusor overactivity; NICE: National Institute for Health and Care Excellence.
NICE guidance:7
• Offer bladder wall injection with botulinum toxin type A to adults:
• with spinal cord disease (for example, spinal cord injury or multiple sclerosis) and
• with symptoms of an overactive bladder and
• in whom antimuscarinic drugs have proved to be ineffective or poorly tolerated
• Before offering bladder wall injection with botulinum toxin type A:
• explain to the person and/or their family members and carers that a catheterisation regimen is needed in most people with neurogenic lower urinary tract dysfunction after treatment, and
• ensure that they are able and willing to manage such a regimen should urinary retention develop after the treatment
NICE [2012] Urinary incontinence in neurological disease. Available from nice.org.uk. All rights reserved. Subject to Notice of rights (link to: https://www.nice.org.uk/terms-and-conditions#notice-of-rights)
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250047.












 With spinal cord disease (for example, spinal cord injury or multiple sclerosis) and
With spinal cord disease (for example, spinal cord injury or multiple sclerosis) and