This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
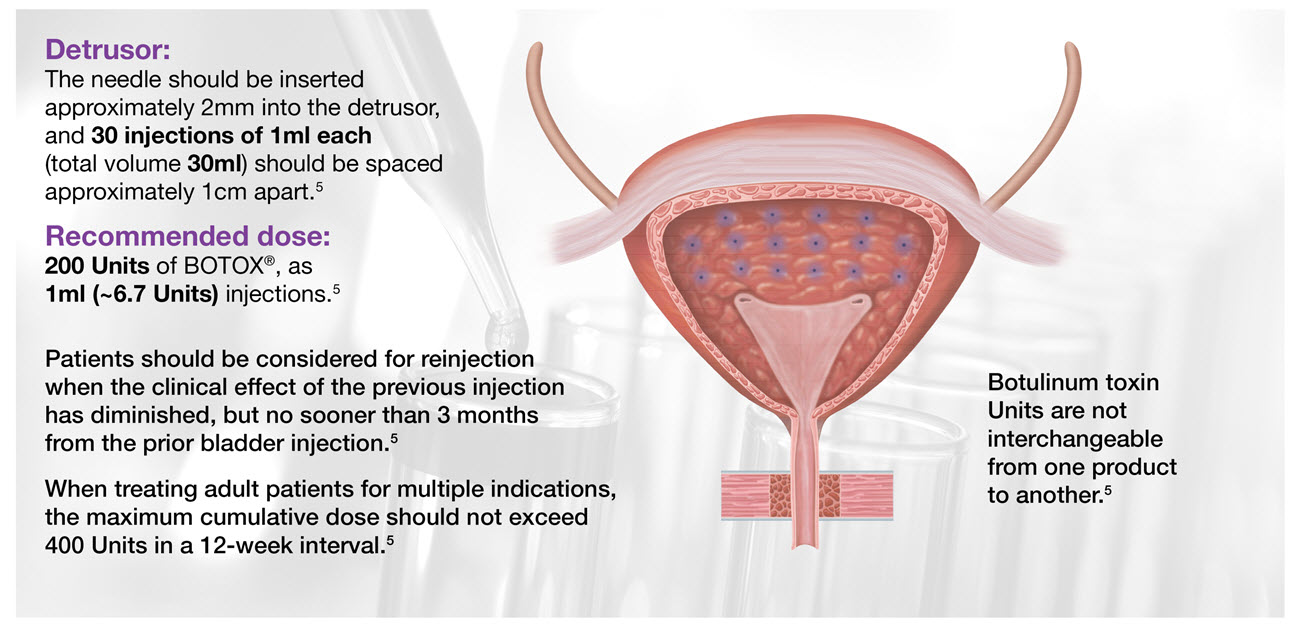
Administering BOTOX® for your patients with neurogenic detrusor overactivity (NDO)
BOTOX® provides approximately 9 months of symptom relief†5
Image adapted from BOTOX® Summary of Product Characteristics5
†As demonstrated by patients who continued into the open label extension study.
BOTOX® in NDO: Treatment benefit sustained over time6
Reductions in mean UI episodes per day at Week 6 after BOTOX® 200U compared with baseline were consistent during the 4 years of treatment (n=122; primary endpoint)6
Adapted from Rovner, 2016.6
Study context: Post-hoc analysis of 227 patients with urinary incontinence due to NDO, who completed 4 years of treatment following an initial 52-week phase 3 trial and then a 3-year open label extension study. Primary efficacy endpoint was change from baseline in UI episodes per day at Week 6.6
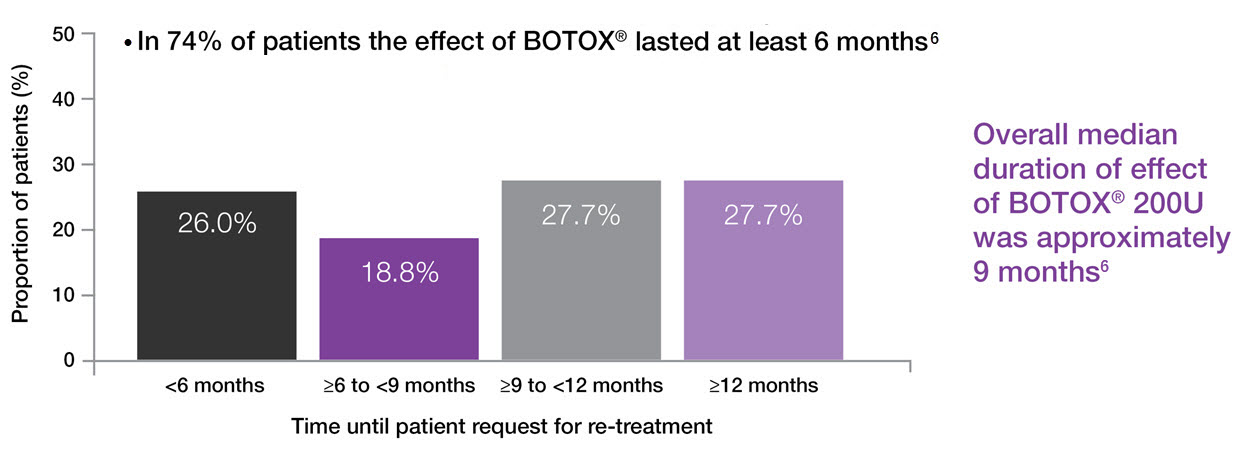
BOTOX® duration of effect6
Adapted from Rovner, 2016.6
Study context: Post-hoc analysis of 227 patients with urinary incontinence due to NDO, who completed 4 years of treatment following an initial 52-week phase 3 trial and then a 3-year open label extension study. Primary efficacy mendpoint was change from baseline in UI episodes per day at Week 6. Overall median duration of effect by time in 112 patients on 200U based on 4-week months. Time to request for re-treatment was calculated from date of patient request for re-treatment in relation to previous treatment date.6
I-QoL: Incontinence-quality of life; NDO: neurogenic detrusor overactivity; SmPC: summary of product characteristics; UI: urinary incontinence.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250048.