This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
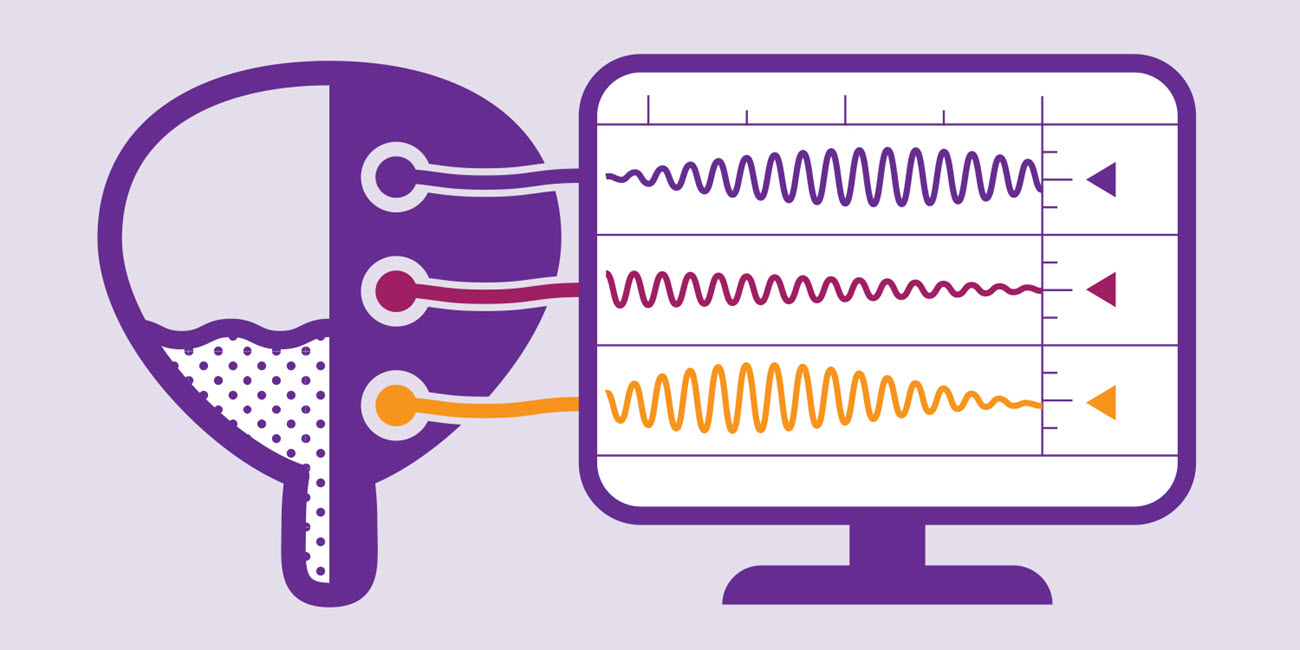
How to diagnose neurogenic detrusor overactivity (NDO)
Early diagnosis and treatment of NDO is essential to prevent irreversible changes within the urinary tract.6
The diagnosis of NDO should include a detailed history, general and neurological physical examination and urinalysis.6–9
Assessment of the patient’s risk factors and quality of life is also required.6 For initial management, basic diagnostic tests should be used to exclude an underlying disease.8
For specialised management, more elaborate assessment is generally required, including imaging, endoscopy and urodynamics.8
 Urodynamics assess filling and storage in the urinary tract and can be used for diagnosis and to direct targeted treatments.6
Urodynamics assess filling and storage in the urinary tract and can be used for diagnosis and to direct targeted treatments.6
 In patients with spinal cord injury, there is a high correlation between functional level of injury and urodynamic abnormalities. Unmanaged high detrusor pressure is a trigger for upper urinary tract injuries leading to deterioration of the upper urinary tract function and kidney failure.10,11
In patients with spinal cord injury, there is a high correlation between functional level of injury and urodynamic abnormalities. Unmanaged high detrusor pressure is a trigger for upper urinary tract injuries leading to deterioration of the upper urinary tract function and kidney failure.10,11
 In patients with multiple sclerosis, urodynamic assessment should be repeated at regular intervals to optimise clinical management, reduce complications, and better enable patients to manage their condition.12
In patients with multiple sclerosis, urodynamic assessment should be repeated at regular intervals to optimise clinical management, reduce complications, and better enable patients to manage their condition.12
NDO: neurogenic detrusor overactivity.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250051.