This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
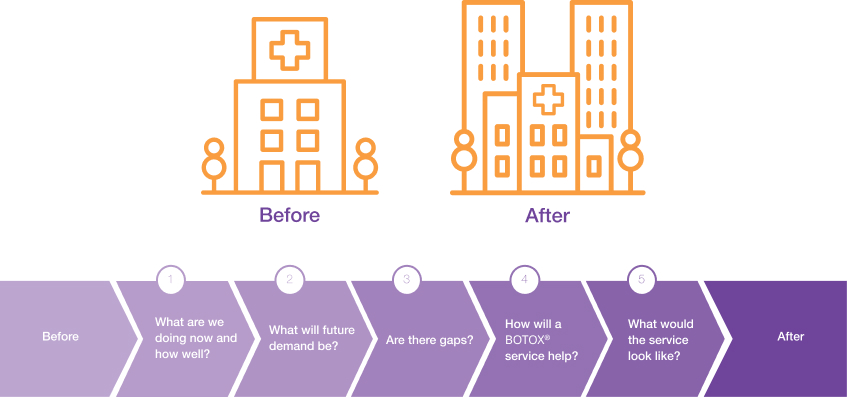
Your vision will describe how the service can improve the quality of care and outcomes for patients with overactive bladder (OAB) or neurogenic detrusor overactivity (NDO). It may also describe what you intend your service to look like in 3–5 years’ time and how your service will help to address any gaps or challenges in the local health environment.
Engagement with key stakeholders involved in the care of patients with OAB or NDO will be essential to shape the vision and planning of your service.
Incorporate intermediate steps towards your goal
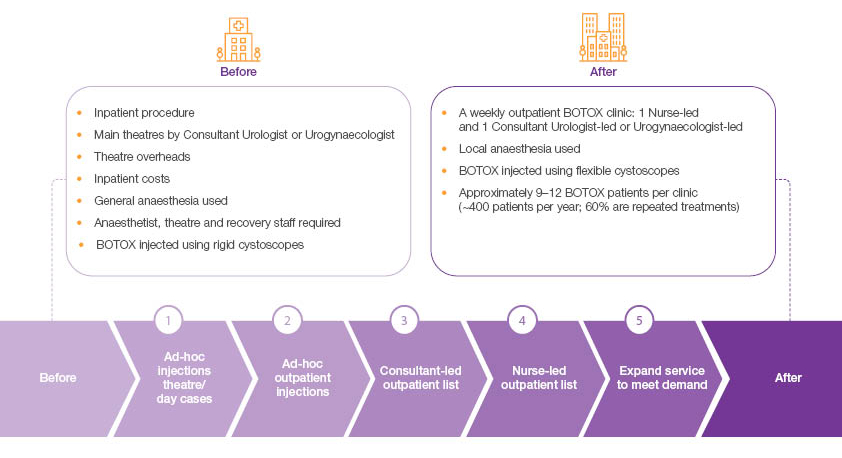
Advantages of the nurse playing a key role, include:
 Nurse specialists are motivated and keen to take on the new role
Nurse specialists are motivated and keen to take on the new role
 It can allow consultant time to be dedicated to emergency care
It can allow consultant time to be dedicated to emergency care
 Nurse-led outpatient clinics can result in improved waiting times for patients, improved quality of life and better patient satisfaction
Nurse-led outpatient clinics can result in improved waiting times for patients, improved quality of life and better patient satisfaction
For further information and examples of good clinical practice in line with GIRFT guidance, please request support via the Contact Us page.
Other considerations in order to achieve your vision for your BOTOX® urology service:
 Operational considerations such as:
Operational considerations such as:
 The procedure itself
The procedure itself
 Equipment
Equipment
 Staff and training
Staff and training
 Available facilities
Available facilities
 Patient scheduling
Patient scheduling
 Creating a patient-centric service: through the provision of pre- and post-procedure patient education and follow-up
Creating a patient-centric service: through the provision of pre- and post-procedure patient education and follow-up
 Securing funding: Gaining financial support for your service is a critical part of the planning process. It is important to:
Securing funding: Gaining financial support for your service is a critical part of the planning process. It is important to:
 develop a business case
develop a business case
 estimate the service income
estimate the service income
 identify and engage with the right stakeholders
identify and engage with the right stakeholders
GIRFT: Getting It Right First Time; NDO: neurogenic detrusor overactivity; OAB: overactive bladder.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here.
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250042.