ADVERSE EVENTS, CONTRAINDICATIONS, SPECIAL WARNINGS & PRECAUTIONS
2,657 PATIENTS WITH MIGRAINE RECEIVED AT LEAST ONE DOSE OF ATOGEPANT IN CLINICAL STUDIES:1
• 1,225 were exposed to atogepant for at least 6 months
• 826 were exposed for 12 months
• In 12-week, placebo-controlled clinical studies, 678 patients received at least one dose of atogepant 60mg once daily, and 663 received placebo
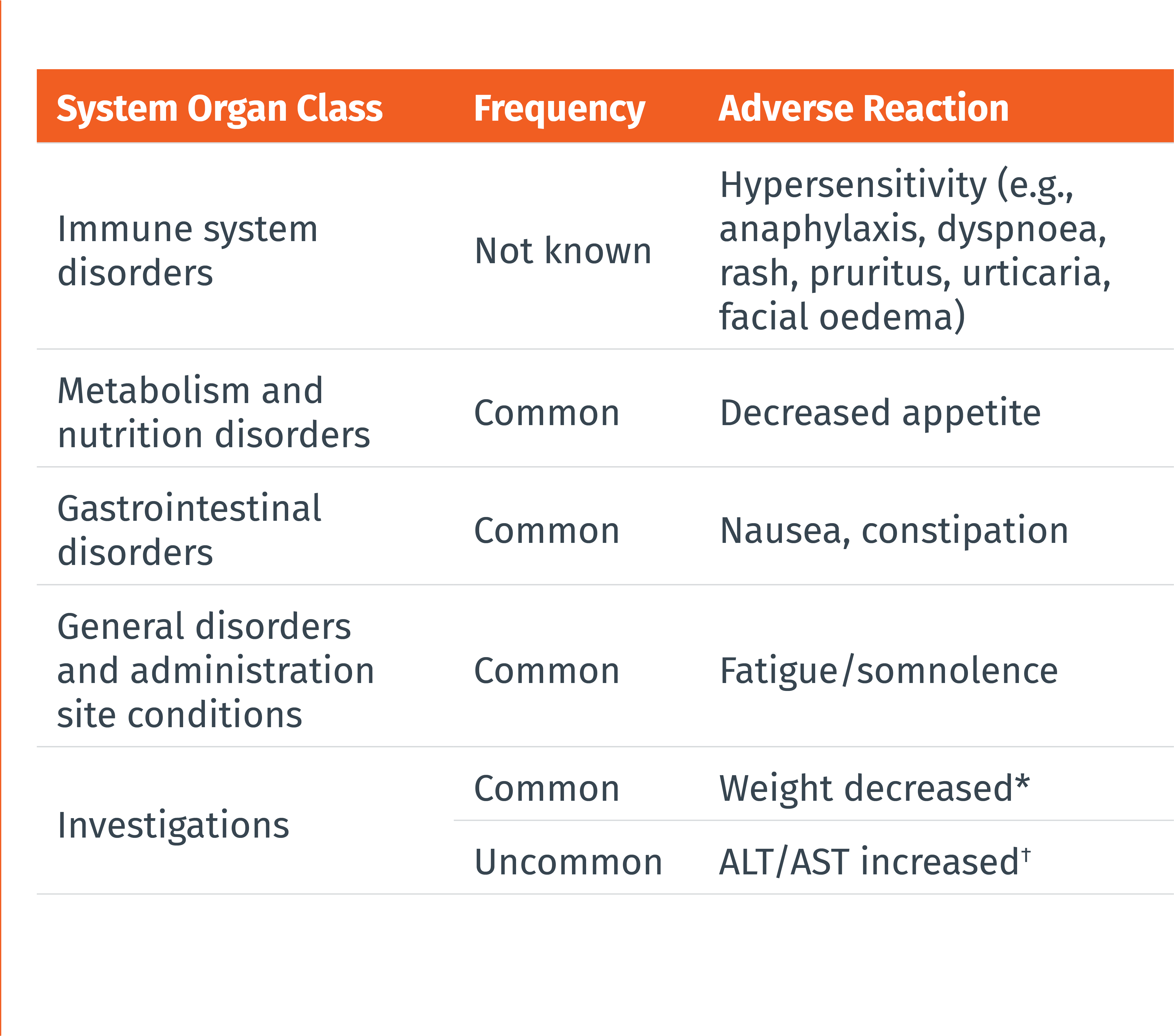
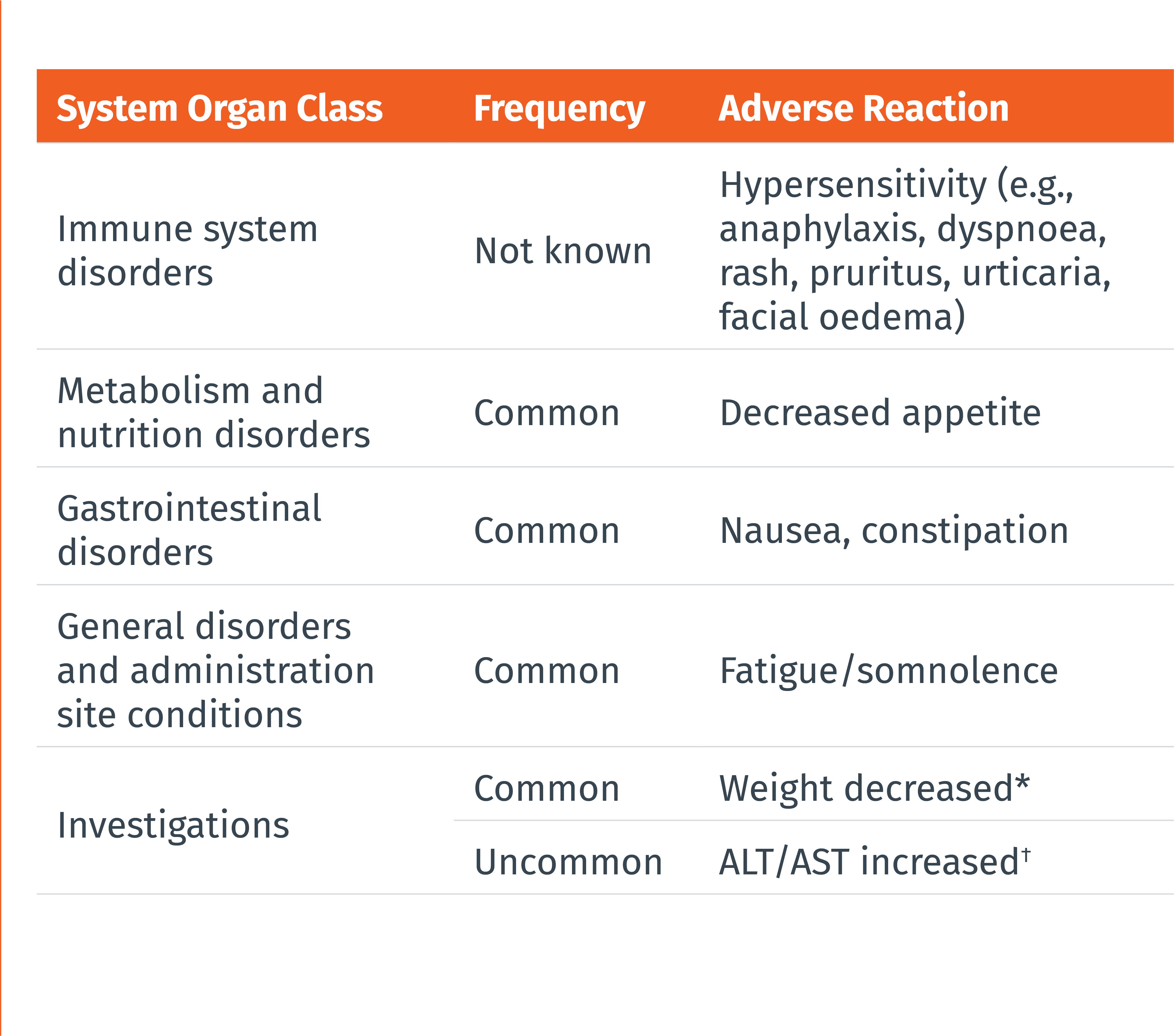
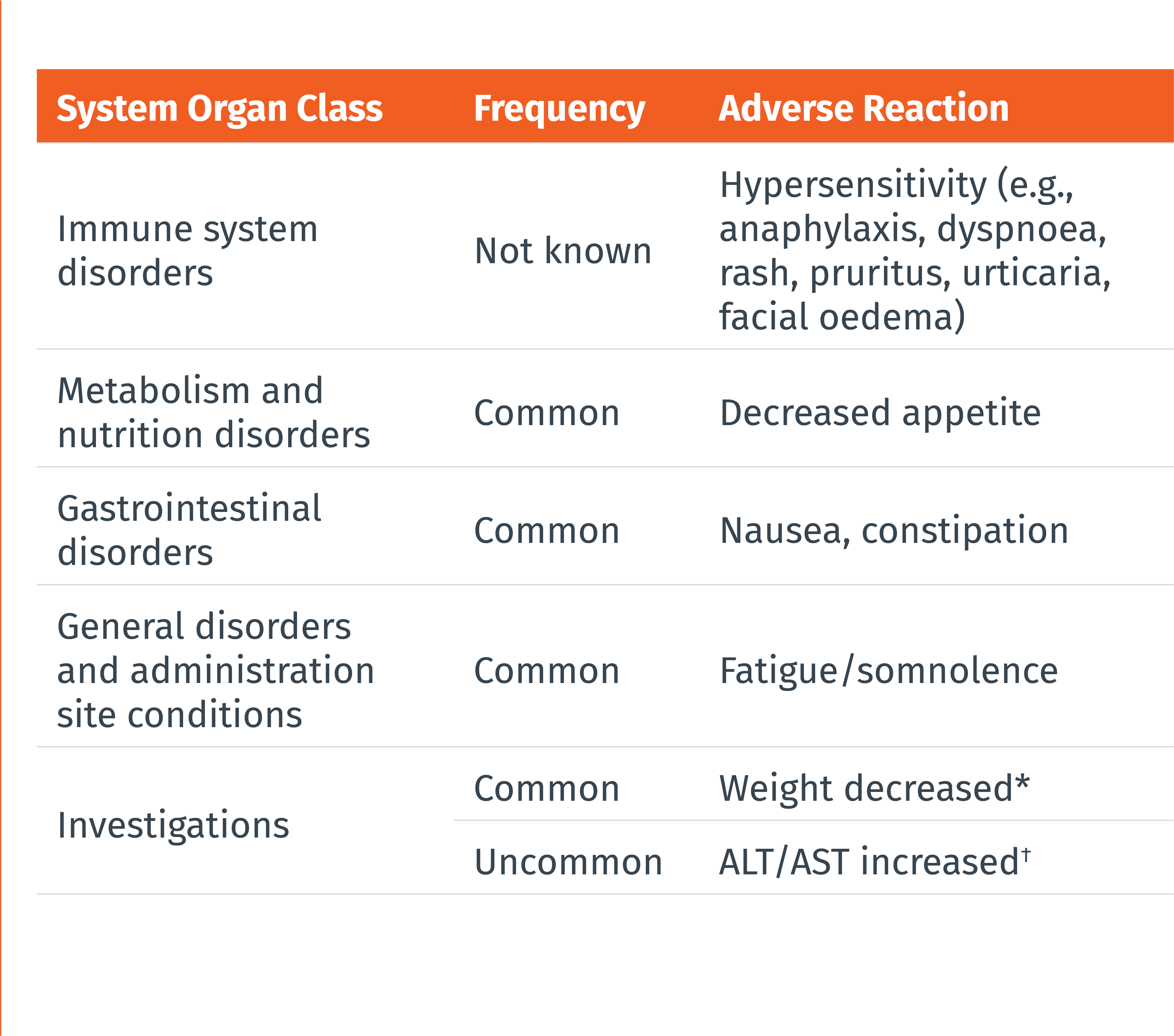
THE MOST COMMONLY REPORTED ADVERSE DRUG REACTIONS WERE:1
• Nausea (9%)
• Constipation (8%)
• Fatigue/somnolence (5%)
• Most of the reactions were mild or moderate in severity
• The adverse reaction that most commonly led to discontinuation was nausea (0.4%)
Frequencies are defined as follows: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1 000 to < 1/100), rare (≥ 1/10 000 to < 1/1 000), very rare (< 1/10 000), or not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in the order of decreasing seriousness.
* Defined in clinical trials as weight decrease of at least 7% at any point. † Cases of ALT/AST elevations (defined as ≥3x upper limit of normal) temporally associated with atogepant were observed in clincal trials, including cases with a potential positive dechallenge history that resolved within 8 weeks of discontinuation. However, the overall frequency of liver enzyme elevations was similar in the atogepant and placebo groups.
SAFETY PROFILE CONTD.
CONTRAINDICATIONS
• Hypersensitivity to the active substance (atogepant) or to any of the excipients listed in section 6.1 of the SmPC.1
SPECIAL WARNINGS AND PRECAUTIONS FOR USE
• Atogepant is not recommended in patients with severe hepatic impairment.1
• Serious hypersensitivity reactions, including anaphylaxis, dyspnoea, rash, pruritus, urticaria, and facial oedema, have been reported with use of AQUIPTA® (see section 4.8 of SmPC). Most serious reactions have occurred within 24 hours of first use, however, some hypersensitivity reactions can occur days after administration.1
• Patients should be warned about the symptoms associated with hypersensitivity. If a hypersensitivity reaction occurs, discontinue AQUIPTA and institute appropriate therapy.1
PREGNANCY AND LACTATION
• There are limited data from the use of atogepant in pregnant women. Atogepant is not recommended during pregnancy and in women of childbearing potential not using contraception.1
• Pharmacokinetic data after single-dose administration showed minimal transfer of atogepant into breast milk (see section 5.2 of the SmPC). There are no data on the effects of atogepant on the breastfed infant or the effects of atogepant on milk production. The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for atogepant and any potential adverse effects on the breastfed infant from atogepant or from the underlying maternal condition.1
References:
1. AQUIPTA® Summary of Product Characteristics (Ireland).
IE-AQP-250020 Date of Preparation: October 2025