Quality of Life
Duodopa® reduces motor symptoms resulting in a sustained improvement in health-related quality of life2
In a study Duodopa® improved HRQoL-related measures from week 4 and throughout the study duration (p<0.001)2
Adapted from Fernandez HH et al. 2015.
A 54-week, international, prospective, open-label study assessed the safety and efficacy of Duodopa® in advanced PD patients with severe motor fluctuations (N=354). Safety was the primary endpoint; for the majority of patients AEs were mild or moderate and transient. Secondary endpoints included 'OFF' time, 'ON' time without troublesome dyskinesia, UPDRS and HRQoL outcomes.2
ADL=activities of daily living; AE=adverse event; EQ-SD=EuroQol-five dimensions; EQ-VAS=EuroQol-visual analogue scale; HRQoL=health-related quality of life; PD=Parkinson's disease; PDQ=Parkinson Disease Questionnaire; UPDRS=Unified Parkinson's Disease Rating Scale.
1. Nyholm D, et al. AAPS J 2013;14:316–23.
2. Fernandez HH, et al. Mov Disord 2015;30(4):500–9.
3. Freire-Alvarez E, et al. Mov Disord. 2021;36(11):2615-23.
Duodopa® improves patients' quality of life1
The GLORIA registry: A 24-month, non-interventional, observational registry study, evaluating the long-term efficacy of Duodopa® on motor and non-motor symptoms, QoL and safety in advanced PD patients (N=375).1
In the primary analysis, from baseline until last visit, Duodopa® significantly improved:1
- Patients' difficulties getting around in public (p<0.001)
- Painful muscle cramps/pain (p=0.002)
- Embarrassment (p<0.001)
Adapted from Antonini A et al. 2017.
PD=Parkinson's disease; PDQ=Parkinson Disease Questionnaire.
1. Antonini A, et al. Parkinsonism Relat Disord 2017;45:13-20 and Supplementary Data.
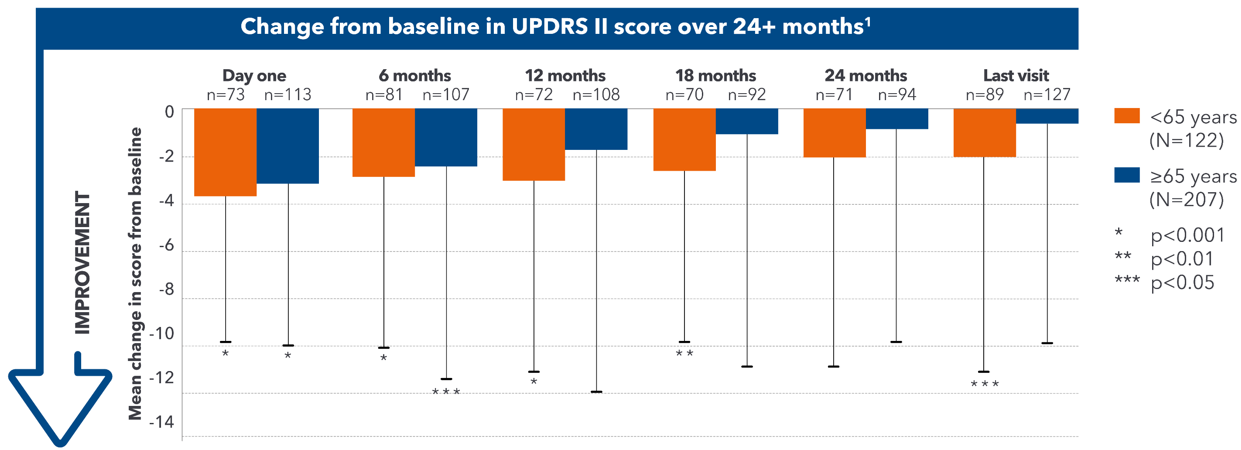
Duodopa® improves patients' activities of daily living1
A post-hoc analysis of the GLORIA registry evaluated whether age and PD duration at therapy initiation influenced the efficacy of Duodopa® on QoL and ADL.1
- Patients demonstrated sustained improvements in ADL, as shown by a reduction in their UPDRS II score1
- While improvements in ADL were observed in patients with longer PD duration (≥10 years), there were also significant improvements in ADL in patients with shorter PD duration (<10 years)1
- In addition to improvements seen in patients ≥65 years old, greater improvements in ADL were seen in patients <65 years old1
- This may provide justification to consider Duodopa® early in advanced PD1
Adapted from Antonini A et al. 2018.
ADL=activities of daily living; PD=Parkinson's disease; QoL=quality of life; UPDRS=Unified Parkinson's Disease Rating Scale.
1. Antonini A, et al. Neurodegener Dis Manag 2018;8(3):161-70.
IE-DUOD-220033. Date of preparation: November 2022.







1.png)