An established and well-understood safety profile1-4
- Generally well-understood safety profile over 17 years since first approval1-4
- Drug-related undesirable effects that occur most frequently with the Duodopa® system include nausea and dyskinesia, urinary tract infection, weight decrease, anxiety, depression, insomnia, Parkinson's disease, orthostatic hypotension, constipation and fall4
- Device- and procedure-related undesirable effects that occur frequently with the Duodopa® system include abdominal pain, complications of device insertion, excessive granulation tissue, incision site erythema, postoperative wound infection, post procedural discharge, procedural pain, and procedural site reaction4
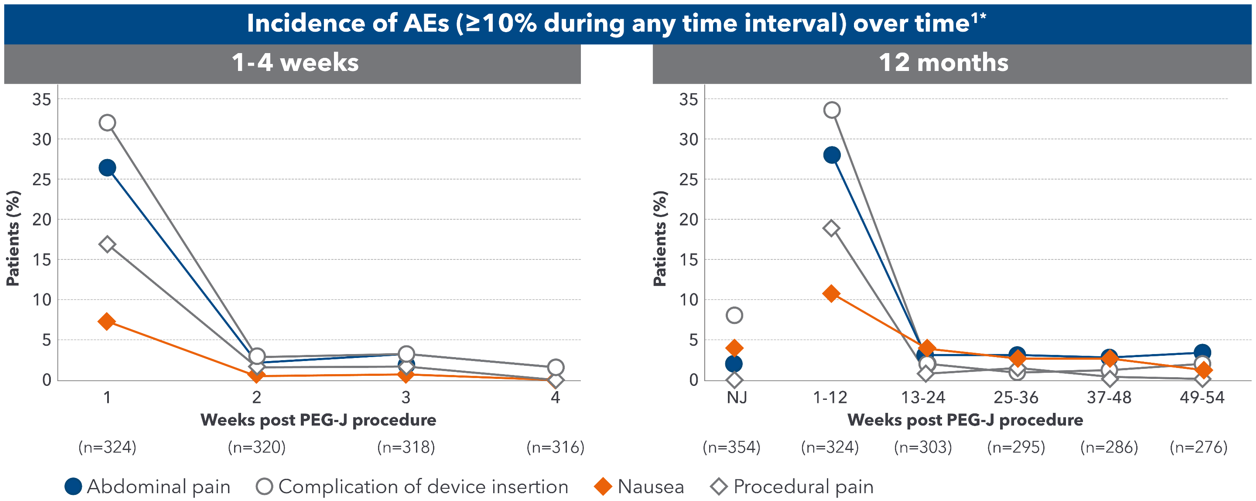
- Most device- and procedure-related adverse reactions to Duodopa® occurred during the first 28 days after the PEG-J procedure4
For full safety information please refer to the Summary of Product Characteristics available at www.medicines.ie
PEG-J=percutaneous endoscopic jejunostomy; PD=Parkinson's disease; HCl=hydrochloride; MAO=monoamine oxidase.
1. Fernandez HH, et al. Mov Disord 2015;30(4):500–9.
2. Antonini A, et al. Parkinsonism Relat Disord 2017;45:13–20.
3. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu301035 (Accessed March 2024).
4. Duodopa® Summary of Product Characteristics, available on www.medicines.ie.
Duodopa® has a generally well-understood safety profile1
- For the majority of patients, AEs were mild (18.5%) or moderate (43.8%) and transient1
- There was a low withdrawal rate (7.6%) due to AEs, most commonly due to complication of insertion of device1
- AEs decreased over time after PEG-J procedure and further decreased over 12 months1
Adapted from Fernandez HH et al. 2015.
A 54-week, international, prospective, open-label study assessed the safety and efficacy of Duodopa® in advanced PD patients with severe motor fluctuations (N=354).
324 (91.5%) of the 354 enrolled patients completed the NJ phase and proceeded to PEG-J
*A single event could be coded to> 1 preferred term.1
AE=adverse event; NJ=nasojejunal; PD=Parkinson's disease; PEG-J=percutaneous endoscopic jejunostomy.
1. Fernandez HH, et al. Mov Disord 2015;30(4):500-9.
Duodopa® is contraindicated in patients with1:
- Hypersensitivity to the active substances or to any of the excipients
- Narrow-angle glaucoma
- Severe heart failure
- Severe cardiac arrhythmia
- Acute stroke
- Non-selective MAO inhibitors and selective MAO type A inhibitors are contraindicated for use with Duodopa® . These inhibitors must be discontinued at least two weeks prior to initiating therapy with Duodopa® . Duodopa® may be administered concomitantly with the manufacturer's recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g., selegiline HCI)
- Conditions in which adrenergics are contraindicated, e.g. pheochromocytoma, hyperthyroidism and Cushing's syndrome
- Because levodopa may activate malignant melanoma, Duodopa® should not be used in patients with suspicious undiagnosed skin lesions or a history of melanoma
For full safety information please refer to the Summary of Product Characteristics available at www.medicines.ie
HCl=hydrochloride; MAO=monoamine oxidase.
1. Duodopa® Summary of Product Characteristics, available on www.medicines.ie.
IE-DUOD-240005. Date of preparation: April 2024.