Note to affiliates: This update to the venetoclax CLL AbbVie Pro site includes a homepage headline, updated CLL14 6-year, CLL13 4-year, MURANO 7-year data sets, and other streamlined content updates. CLL 13 4-year update reflects the CLL13 data from the Lancet Oncology publication. The CLL14 6-year and MURANO 7-year data have been updated based on the EHA 2023 abstracts. For countries that cannot use these data sets, please follow local regulations and MRLO guidance, and revert to CLL14 5-year and MURANO 5-year published data from the product label.
Primary analysis in ITT population for VEN+O vs O+Clb1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.35; 95% CI: 0.23–0.53 [P<0.0001]).
| • | Median follow-up of 28 months |
Additional analyses:
6-year PFS estimate (INV-assessed)2‡: 53% vs 22% (HR=0.40; 95% CI: 0.31–0.52) after 5 years off treatment.
| • | Median PFS of 76.2 months with VEN+O vs 36.4 months with O+Clb |
INV-assessed complete remission (CR/CRi)1: 50% vs 23% (P<0.0001).
| • | ORR: 85% (95% CI: 79.2–89.2) vs 71% (95% CI: 64.8–77.2 [P=0.0007]) |
Primary analysis in ITT population for VEN+R vs BR1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.17; 95% CI: 0.11–0.25 [P<0.0001]).
| • | Median follow-up of 23.8 months |
Additional analyses:
7-year PFS estimate (INV-assessed)3‡: 23% (HR=0.23; 95% CI: 0.18–0.29) vs NE after ~5 years off treatment.
| • | Median PFS of 54.7 months with VEN+R (95% CI: 52.3–59.9) vs 17.0 months with BR (95% CI: 15.5–21.7) |
INV-assessed complete remission (CR/CRi)1‡: 27% vs 8%.
| • | ORR: 93% (95% CI: 88.8–96.4) vs 68% (95% CI: 60.6–74.2) |
*See full dosing information for VEN+O and for VEN+R in the dosing and administration section.
†Primary endpoint.
‡Results are descriptive only.
1L=first line; CLL=chronic lymphocytic leukaemia; VEN+O=VENCLYXTO + obinutuzumab; ITT=intent to treat; O+Clb=obinutuzumab + chlorambucil; INV=investigator; PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete bone marrow recovery; ORR=overall response rate; 2L+=second line + later lines of therapy; VEN+R=VENCLYXTO + rituximab; BR=bendamustine + rituximab; NE=not evaluable.
VENCLYXTO in combination with obinutuzumab is indicated for the treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL).
VENCLYXTO in combination with rituximab is indicated for the treatment of adult patients with CLL who have received at least one prior therapy.
VENCLYXTO monotherapy is indicated for the treatment of CLL:
| • | in the presence of 17p deletion or TP53 mutation in adult patients who are unsuitable for or have failed a B‑cell receptor pathway inhibitor, or |
| • | in the absence of 17p deletion or TP53 mutation in adult patients who have failed both chemoimmunotherapy and a B‑cell receptor pathway inhibitor. |
Note to affiliates: For countries that cannot yet promote on 5-year data, please change to 3-year follow-up data. Please see the VENCLYXTO SmPC for full prescribing and safety information. Discussions are ongoing on the use of CLL14 TTNT for promotion. Additional guidance is expected in the future.
USE VENCLYXTO AT THE FIRST INDICATED OPPORTUNITY IN YOUR PATIENTS WITH CLL1
DEEP RESPONSE*
| • | uMRD at EoT (PB)†: 76% (95% CI: 69–81) vs 35% (95% CI: 29–42) (P<0.0001) |
| • | INV-assessed complete remission (CR/CRi): 50% vs 23% (P<0.0001) |
| • | uMRD at EoCT (PB)†‡: 62% (95% CI: 55.2–69.2) vs 13% (95% CI: 8.9–18.9) |
| • | INV-assessed complete remission (CR/CRi)‡: 27% vs 8% |
SUSTAINED§ EFFICACY WITH PROLONGED TIME OFF TREATMENT
| • | Primary analysis (INV-assessed PFS): Reduced risk of progression or death (HR=0.35; 95% CI: 0.23–0.53 [P<0.0001]). Median follow-up of 28 months |
| • | 5-year PFS estimate (INV-assessed)‡: 63% vs 27% (HR=0.35; 95% CI: 0.26–0.46) after 4 years off treatment |
| - | Median PFS NR in VEN+O vs 36.4 months with O+Clb2 |
| • | 5-year TTNT rate: 72% vs 43% (HR=0.42; 95% CI: 0.31–0.57) after 4 years off treatment. Results are descriptive, not tested for statistical significance |
| • | Primary analysis (INV-assessed PFS): Reduced risk of progression or death (HR=0.17; 95% CI: 0.11–0.25 [P<0.0001]). Median follow-up of 23.8 months |
| • | Median PFS (INV-assessed)‡: 54 months (~4.5 years) vs 17 months (~1.5 years) (HR=0.19; 95% CI: 0.15–0.26) after ~3 years off treatment |
| • | Median TTNT: 58 months vs 24 months (HR=0.26; 95% CI: 0.20–0.35) after ~3 years off treatment3 |
| • | 5-year OS estimate‡: 82% vs 62% (HR=0.40; 95% CI: 0.26–0.62) |
MANAGEABLE SAFETY
| • | Most common ARs (≥20%): neutropaenia, diarrhoea, and upper respiratory tract infection |
| • | The most frequently reported serious ARs (≥2%): pneumonia, sepsis, febrile neutropaenia, and TLS, including fatal events and renal failure during analysis, have occurred. Serious infections including events of sepsis with fatal outcome have been reported |
| • | 16% of patients discontinued treatment due to ARs in both the CLL14 and MURANO studies |
*Deep response as measured by uMRD or CR.
†MRD was evaluated using ASO-PCR (CLL14 and MURANO) and/or flow cytometry (MURANO). In PB, the cutoff for an undetectable (negative) status was <1 CLL cell per 104 leukocytes.
‡Results are descriptive.1,2
§Sustained off-treatment response based on 5-year (CLL14) and 5-year (MURANO) updated efficacy analyses.1,2
NR=not reached; TTNT=time to next anti-leukaemic treatment; OS=overall survival; AR=adverse reaction; TLS=tumour lysis syndrome.
Note to affiliates: For countries that choose to incorporate the CLL13 study data found in the Optional Pages of this CVA, this "Importance of uMRD" content must be deleted. Countries can determine during localisation which page is a priority for promotion.
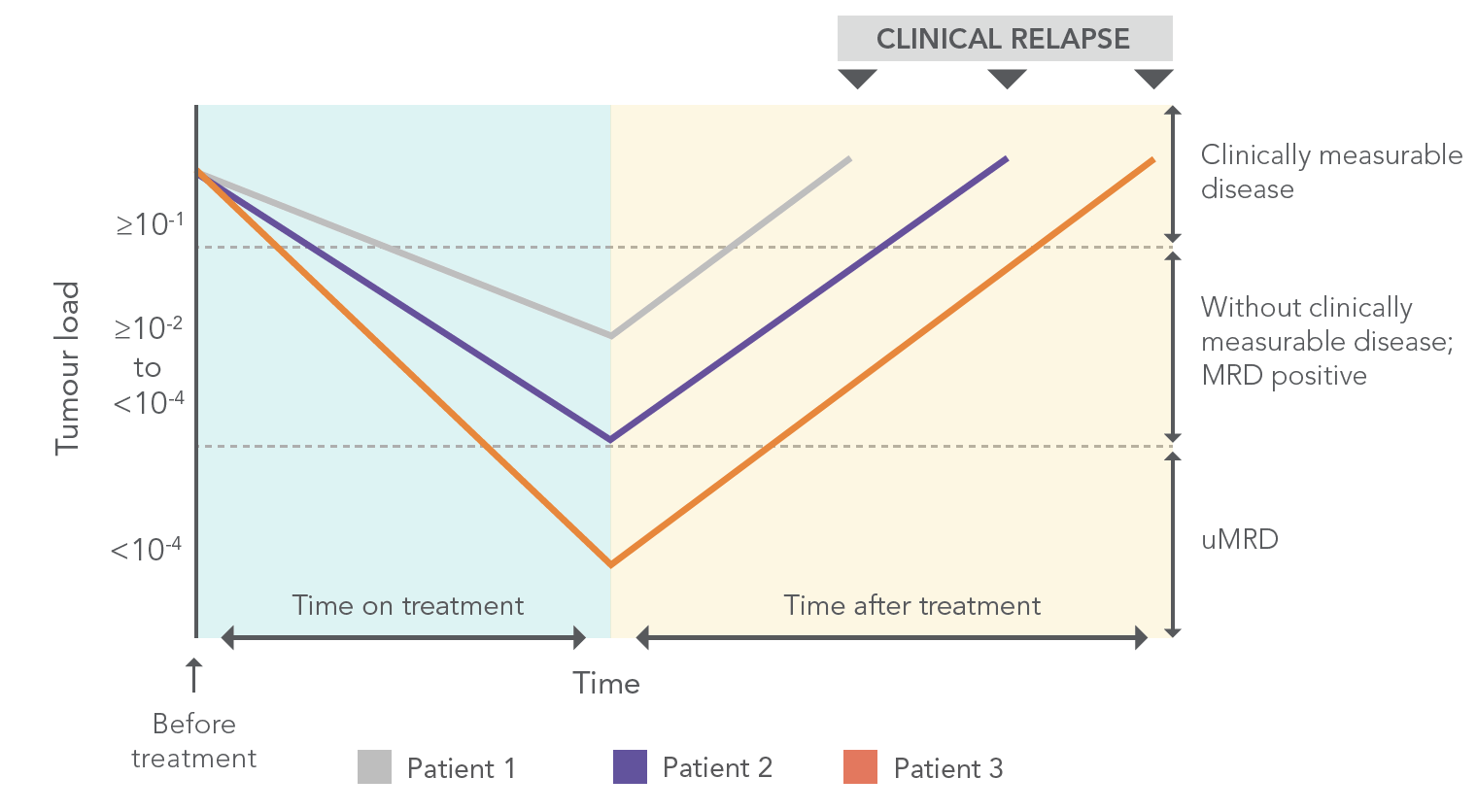
ACHIEVING uMRD* IS IMPORTANT AND MAY BE ASSOCIATED WITH IMPROVED OUTCOMES4,5
REPRESENTATION OF HYPOTHETICAL SCENARIOS OF LEUKAEMIA CELL BURDEN CHANGES IN RESPONSE TO TREATMENT6
Figure adapted from: Böttcher S, et al. 2013.
*uMRD=undetectable minimal residual disease.
VENCLYXTO REGIMENS ARE THE ONLY CHEMO-FREE, FIXED-DURATION REGIMENS THAT CAN BE COMPLETED IN 1 OR 2 YEARS1*
*1-year VEN+O regimen or approximate 2-year VEN+R regimen following 5-week dose-titration schedule.
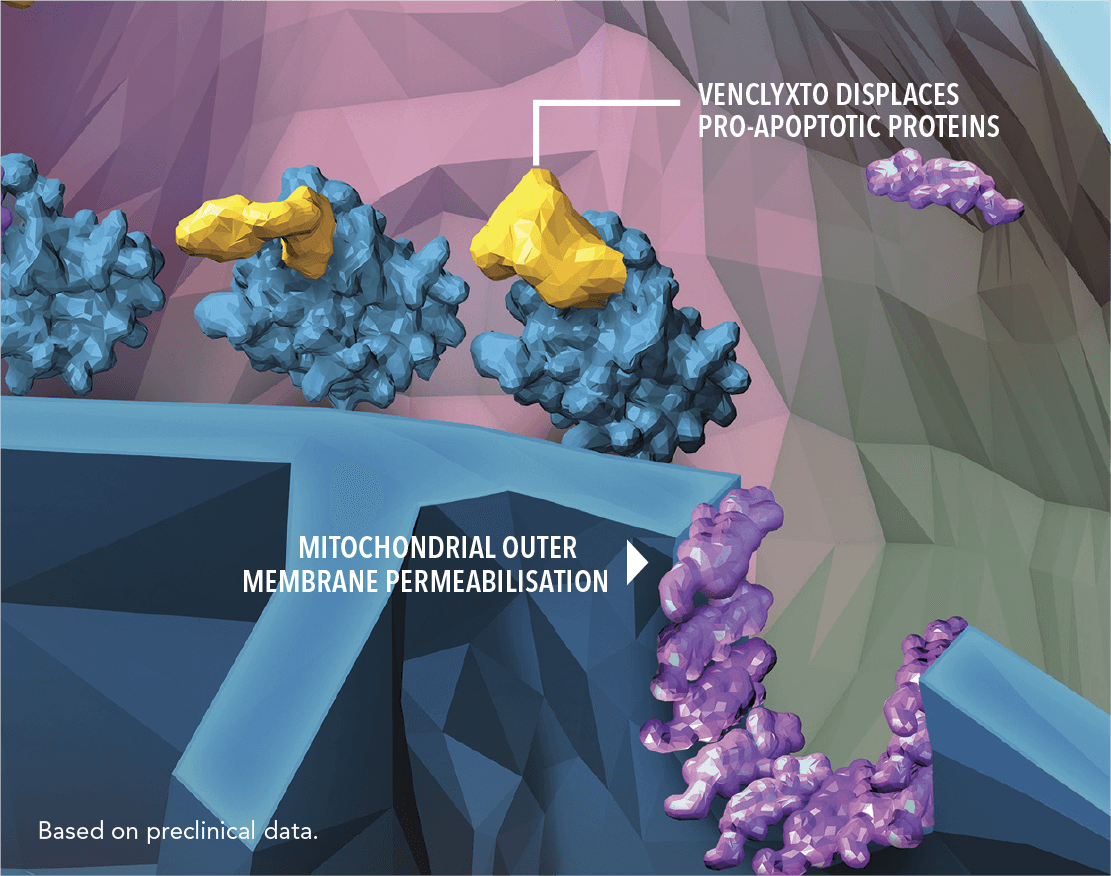
VENCLYXTO IS A FIRST-IN-CLASS, SELECTIVE ORAL BCL-2 INHIBITOR THAT HELPS TO RESTORE THE PROCESS OF APOPTOSIS IN CLL CELLS1
| • | VENCLYXTO selectively binds to anti-apoptotic BCL-2 proteins |
| • | This leads to displacement of pro-apoptotic proteins |
| • | The pro-apoptotic proteins can then dimerize on the mitochondrial membrane, leading to apoptosis |
MOA=mechanism of action; BCL-2=B-cell lymphoma 2.
[Placeholder for safety balance required by local regulations]
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
References: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. 2. Al‑Sawaf O, Zhang C, Robrecht S, et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 5-year results of the randomized CLL14 study. HemaSphere. 2022;6:(S3):49-50. 3. Seymour JF, Kipps TJ, Eichhorst B, et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood. 2022. https://doi.org/10.1182/blood.2021015014. Published online May 5, 2022. 4. Thompson PA, Wierda WG. Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood. 2016;127(3):279-286. 5. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745-2760. 6. Böttcher S, Hallek M, Ritgen M, Kneba M. The role of minimal residual disease measurements in the therapy for CLL: is it ready for prime time? Hematol Oncol Clin North Am. 2013;27(2):267-288.