SKYRIZI: DEMONSTRATED SUPERIORITY
at achieving complete skin clearance in PsO versus 3 agents in different biologic classes
Durable, complete clearance
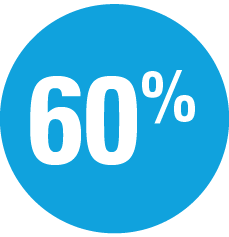
Superior to ustekinumab
at Week 16 and Week 52: UltIMMa pivotal trials (P<0.001)1,4
- 2x the percentage of patients achieved PASI 100 at Week 16 and Week 52 with SKYRIZI (UltIMMa-2)
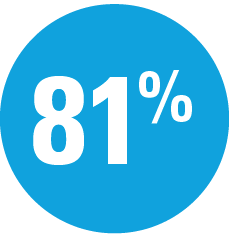
Superior to secukinumab
at Week 52: IMMerge assessor-blinded Phase 3b trial (P<0.001)11,12
Among adalimumab intermediate responders (PASI 50 to <PASI 90) who were rerandomized at Week 16:
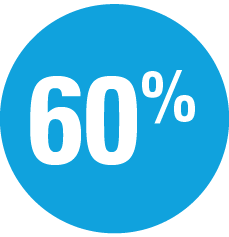
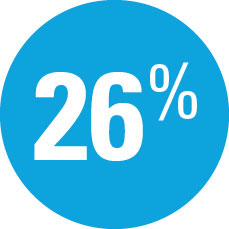
| — | Week 44: 40% SKYRIZI vs 7% adalimumab achieved PASI 100 |
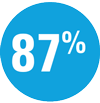
| — | Week 44: 66% SKYRIZI vs 21% adalimumab achieved PASI 90† |
| * | Co-primary endpoint. Note: Some studies were designed with primary endpoints in twoparts (Part A and Part B), per protocol. All other endpoints were ranked secondary. |
| † | Primary endpoint. All other endpoints were ranked secondary. |
NO DOSE ADJUSTMENT REQUIRED, regardless of baseline characteristics, including BMI and weight1,14,15†
- 1 injection/dose for both the SKYRIZI prefilled pen and syringe
- SKYRIZI is dosed 150 mg (one 150-mg subcutaneous injection) at Week 0, Week 4, and every 12 weeks thereafter1
| * | Maintenance dosing (one 150-mg subcutaneous injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. |
| † | Numeric trends toward less efficacy were observed in clinical trials in patients weighing more than 130 kg. However, this observation is based on a limited number of subjects. |
- Consistent AEs of special interest in PsO in a pooled analysis from the first 16 weeks of treatment through 7.8 years16,17*
- PsO safety profile similar to ustekinumab through Week 52 during RCTs1,4
- Studied in >3,500 PsO patients across ~12,000 PYs in an OLE16
PsA safety profile consistent with the safety profile observed in PsO1
| * | Integrated all-risankizumab safety data set from 20 completed or ongoing Phase 1–4 risankizumab clinical trials in plaque psoriasis (data cutoff March 25, 2022): Median duration of treatment was 4.2 years (ranging from 3 days to 7.8 years). |
UltIMMa-1 and UltIMMa-2 replicate Phase 3 study designs1,4
UltIMMa-1/2 were two replicate 52-week, randomized, placebo-controlled comparative studies of SKYRIZI vs ustekinumab in adult patients with moderate to severe chronic plaque psoriasis.1,4
PASI 90 and PASI 100 clearance of psoriatic lesions at Week 52 vs ustekinumab were ranked secondary endpoints.
Data analysis: Missing data were imputed as nonresponders (NRI) for categorical endpoints and by last observation carried forward for continuous endpoints.
Co-primary endpoints: Proportion of patients who achieved PASI 90 response and an sPGA score of clear or almost clear (sPGA 0 or 1) at Week 16 vs placebo.
Ranked secondary endpoints: All 15 secondary ranked endpoints vs placebo and/or ustekinumab at Week 16 and/or Week 52 were met in both UltIMMa-1 and UltIMMa-2 (P<0.0001).
Dosing: SKYRIZI 150 mg (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter. Ustekinumab 45 mg or 90 mg (weight-based per label). Ustekinumab was dosed every 12 weeks after 2 starter doses at Week 0 and Week 4.
IMMerge SKYRIZI vs secukinumab assessor-blinded, head-to-head study design11,12
A Phase 3b, multicenter, randomized, open-label, efficacy assessor-blinded, active-comparator study designed to evaluate the safety and efficacy of SKYRIZI compared to secukinumab in adult patients with moderate to severe plaque psoriasis.
Patients were randomized 1:1 to SKYRIZI (n=164) (150 mg), given as two 75-mg subcutaneous injections at baseline, 4 weeks later, and every 12 weeks thereafter, or secukinumab (n=163) (300 mg) given as two 150-mg subcutaneous injections, at baseline, Weeks 1, 2, 3, and 4, and then every 4 weeks thereafter.
Safety was assessed in all patients.
Data analysis: Missing data were imputed as nonresponders (NRI) for all primary and ranked secondary endpoints.
Primary endpoints:
• PASI 90 at Week 52 (superiority)
• PASI 90 at Week 16 (noninferiority)
Ranked secondary endpoints:
• PASI 100 at Week 52
• PASI 75 at Week 52
• sPGA 0/1 at Week 52
IMMvent pivotal Phase 3 study design13
A 44-week, randomized comparative study vs adalimumab in adult patients with moderate to severe chronic plaque psoriasis (N=605). IMMvent was powered to show superiority of SKYRIZI over adalimumab in achieving:
• PASI 90 response and sPGA scores of clear or almost clear at Week 16
• PASI 90 response at Week 44 after switching from adalimumab to SKYRIZI vs continuing adalimumab among patients with a ≥PASI 50 to <PASI 90 response after 16 weeks of adalimumab treatment
Primary endpoint after rerandomization: PASI 90 at Week 44 (rerandomized patients)
Secondary endpoints:
• PASI 75 at Week 16
• PASI 100 at Weeks 16 and 44 (rerandomized patients only at Week 44)
Part A (baseline to Week 16): Patients received either SKYRIZI 150 mg or adalimumab (80 mg at baseline, 40 mg at Week 1, and then once every 2 weeks).
Part B (Weeks 16 to 44): The SKYRIZI group continued on treatment. For the adalimumab group, treatment regimen was dependent on the level of PASI response:
• PASI <50 switched to SKYRIZI
• PASI 50 to <PASI 90 (rerandomized to either SKYRIZI or adalimumab)
• ≥PASI 90 continued with adalimumab
Adalimumab patients who switched to risankizumab in Part B were dosed at Weeks 16, 20, and 32.
Find out more about SKYRIZI
References
- SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; February 2023.
- Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69-78. doi:10.1007/s00403-015-1613-8
- Ryan C, Puig Z, Zema C, et al. Incremental benefits on patient-reported outcomes for achieving PASI 90 or PASI 100 over PASI 75 in patients with moderate to severe psoriasis. Poster presented at: 2018 European Academy of Dermatology and Venerology (EADV) Congress; September 12–16, 2018; Paris, France. Poster 2002.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double blind, randomised, placebo controlled and ustekinumab controlled phase 3 trials. Lancet. 2018;392(10148):650-661. doi:10.1016/S0140-6736(18):31713-6
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645-648. doi:10.1111/jdv.12817
- Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77-82.e7. doi:10.1016/j.jaad.2016.03.026
- Gooderham MJ, Papp KA, Lynde CW. Shifting the focus – the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Derm Venereol. 2018;32(7):1111-1119. doi:10.1111/jdv.14868
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature Rev Immunol. 2014;14(9):585-600. doi:10.1038/nri3707
- Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/TH17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616-1626. doi:10.1111/jdv.14433
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71(1):141-150. doi:10.1016/j.jaad.2013.12.036
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50-59. doi:10.1111/bjd.19341
- Risankizumab versus secukinumab for subjects with moderate to severe plaque psoriasis. ClinicalTrials.gov identifier: NCT03478787. https://clinicaltrials.gov /ct2/show/NCT03478787. Updated January 6, 2023. Accessed June 7, 2023.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576-586. doi:10.1016/S0140-6736(19)30952-3
- Leonardi C, Gordon K, Longcore M, Gu Y, Puig L. Weight-based analysis of psoriasis area and severity index improvement at 52 weeks of risankizumab or ustekinumab treatment: an integrated analysis of patients with moderate-to-severe plaque psoriasis. Poster presented at: 24th World Congress of Dermatology (WCD); June 10–15, 2019; Milan, Italy. Poster 5248.
- Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830-2838. doi:10.1111/jdv.16521
- Gordon KB, Blauvelt A, Coates L, et al. Risankizumab long-term safety in patients with psoriatic disease: integrated analyses of data from psoriasis and psoriatic arthritis clinical trials. Poster presented at the 31st Congress of the European Academy of Dermatology and Venereology (EADV 2022); September 7-10, 2022; Milan, Italy. Poster 1607.
- Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186(3):466-475. doi:10.1111/bjd.20818
- HUMIRA [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; October 2022.