SKYRIZI: An IL-23/p19 inhibitor2-7
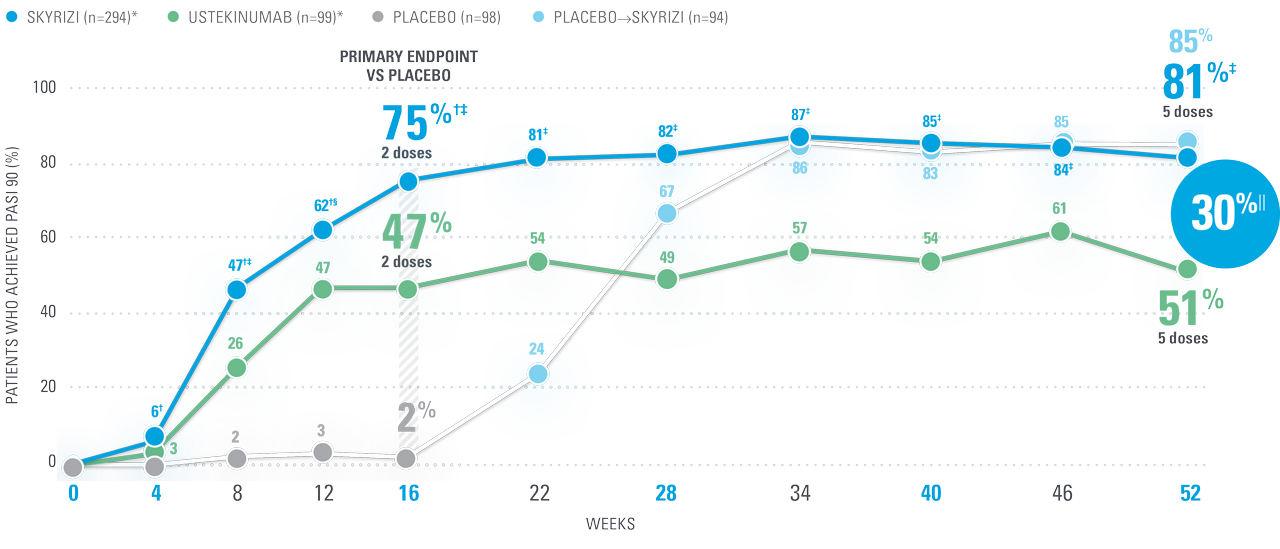
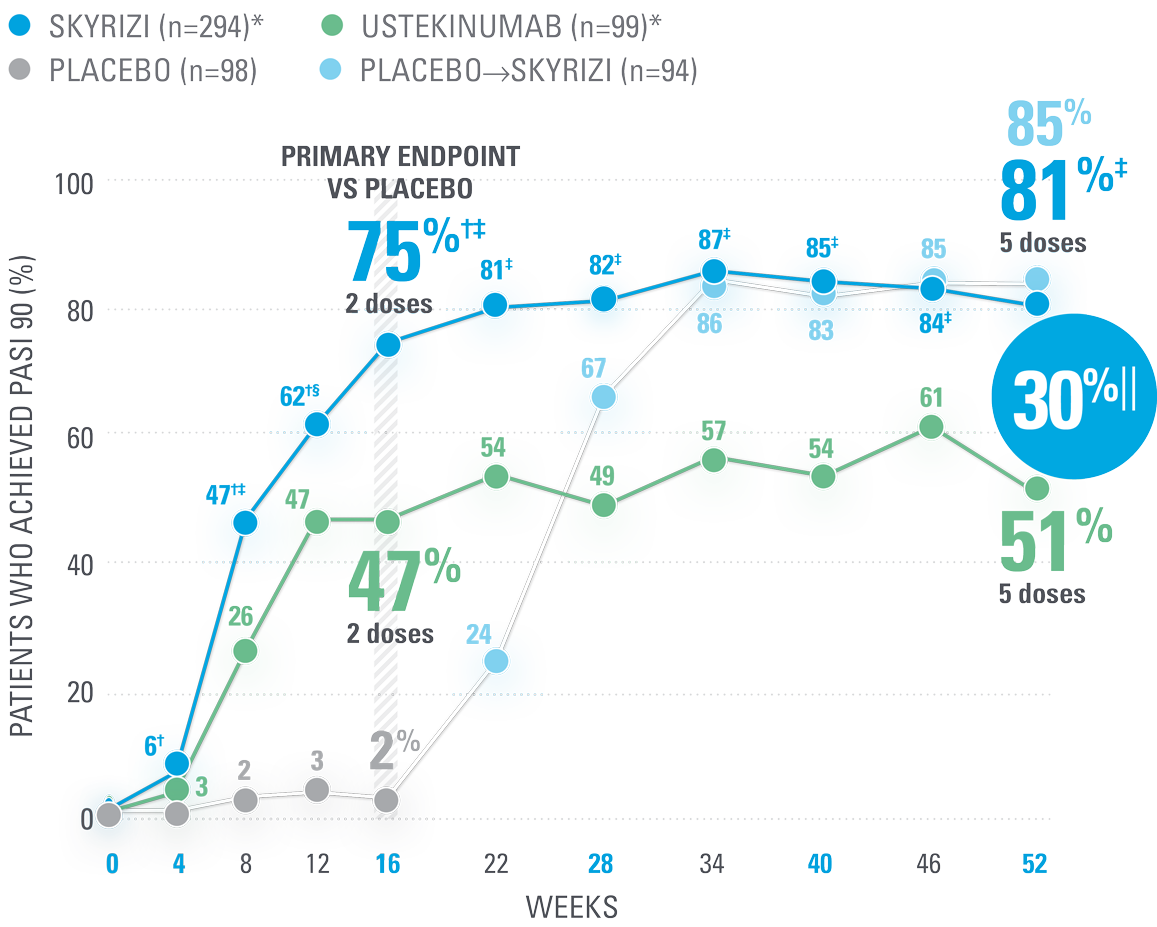
UltIMMa-2 PASI 90
Nothing less than the opportunity for high levels of DURABLE CLEARANCE IN PsO1,8
Superior to ustekinumab:
81% of SKYRIZI patients achieved PASI 90 at Week 52 (NRI)1,8
| * | SKYRIZI doses denoted in blue: Participants received 150 mg SKYRIZI (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter. Ustekinumab 45 mg or 90 mg (weight-based per label). Ustekinumab was dosed every 12 weeks after 2 starter doses at Week 0 and Week 4. |
| † | P<0.0001 vs placebo8 |
| ‡ | P<0.0001 vs ustekinumab8 |
| § | P=0.0107 vs ustekinumab8 |
| || | 30% absolute difference in patients achieving PASI 90 at Week 52 with SKYRIZI (95% CI: adjusted difference; 19.6, 40.9).8 |
Co-primary endpoint: 84% of patients achieved sPGA 0/1 at Week 16 with SKYRIZI vs 5% placebo (P<0.001).1
UltIMMa-1 and UltIMMa-2 replicate Phase 3 PsO study designs1,8
PASI 90 and PASI 100 clearance of psoriatic lesions at Week 52 vs ustekinumab were ranked secondary endpoints.
Data analysis: Missing data were imputed as nonresponders (NRI) for categorical endpoints and by last observation carried forward for continuous endpoints.
Co-primary endpoints: Proportion of patients who achieved PASI 90 response and an sPGA score of clear or almost clear (sPGA 0 or 1) at Week 16 vs placebo.
Ranked secondary endpoints: All 15 secondary ranked endpoints vs placebo and/or ustekinumab at Week 16 and/or Week 52 were met in both UltIMMa-1 and UltIMMa-2 (P<0.0001).