SIMPLICITY
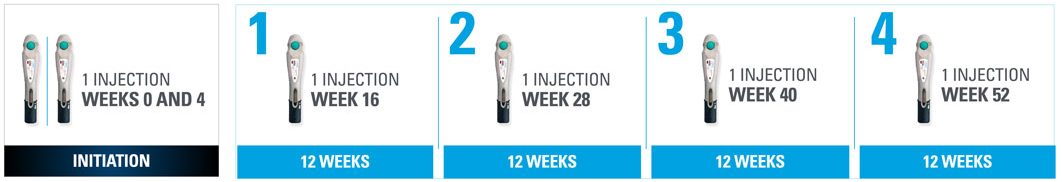
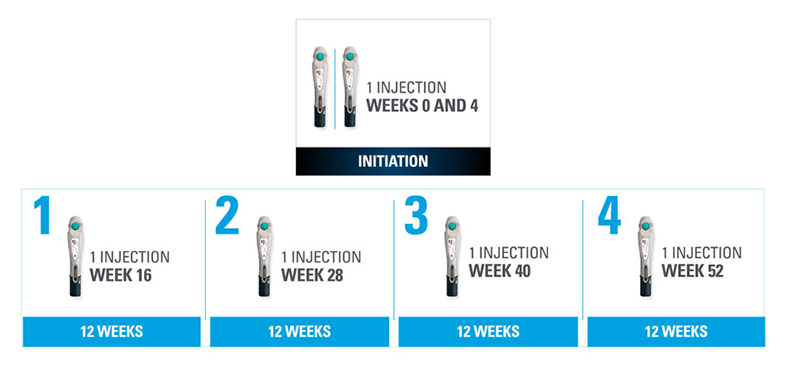
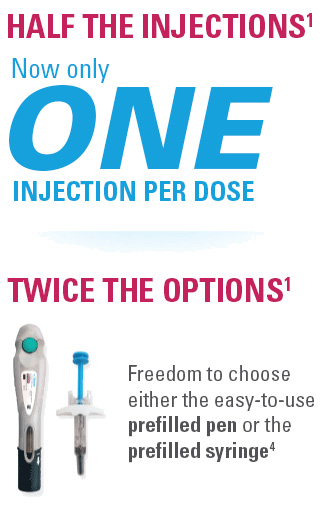
Nothing more than 4 INJECTIONS PER YEAR after initiation doses for both PsO and PsA patients*
- NO DOSE ADJUSTMENT required regardless of baseline characteristics, including BMI and weight1-3†
- SKYRIZI is dosed 150 mg (one 150-mg subcutaneous injection) at Week 0, Week 4, and every 12 weeks thereafter1
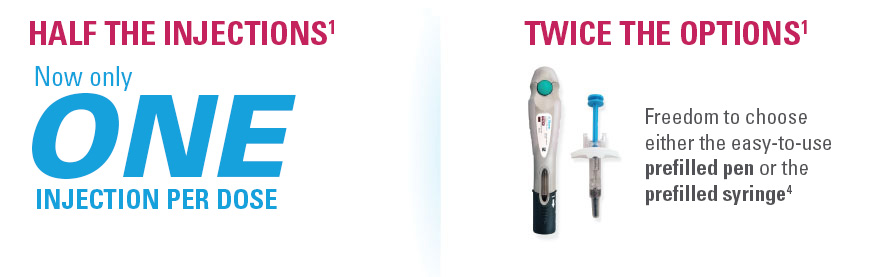
- 1 injection/dose for both the SKYRIZI prefilled pen and prefilled syringe
| * | Maintenance dosing (1 injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. |
| † | Risankizumab clearance and volume of distribution increase as body weight increases, which may result in reduced efficacy in subjects with high body weight (>130 kg). However, this observation is based on a limited number of subjects. |
SKYRIZI one injection per dose:
SAME EFFICACY AND SAFETY PROFILE
Same active ingredient | Demonstrated bioequivalence
NOW EVEN SIMPLER WITH
SKYRIZI 150 mg bioequivalence data1
Bioequivalence was demonstrated between a single SKYRIZI 150-mg injection and two SKYRIZI 75-mg injections in a prefilled syringe. Bioeqivalence was also demonstrated between SKYRIZI 150 mg in a prefilled syringe and a prefilled pen.
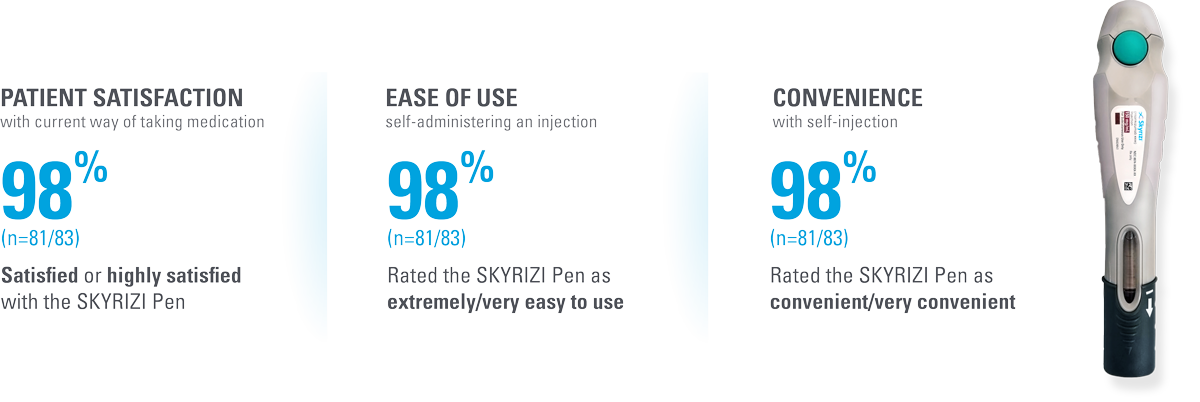
The SKYRIZI prefilled pen scores high across multiple measures of patient satisfaction4*†
| * | Patient satisfaction with self-injection was measured at Week 28 (4 injections total) in patients using the SKYRIZI Pen by the Self-Injection Assessment Questionnaire (SIAQ), a validated instrument for measuring patient feelings and experiences with self-injection. Score measures proportion of subjects achieving the best two categories in post-dose SIAQ by visit (observed cases; intention-to-treat population).4 |
| † | Patient satisfaction was also assessed for (n=83, observed cases): • Satisfied with frequency of injection (97.6%) • Satisfied with duration of injection (96.4%) • Continue self-injecting your medication (100.0%) • Continue self-injecting at home (97.6%) |
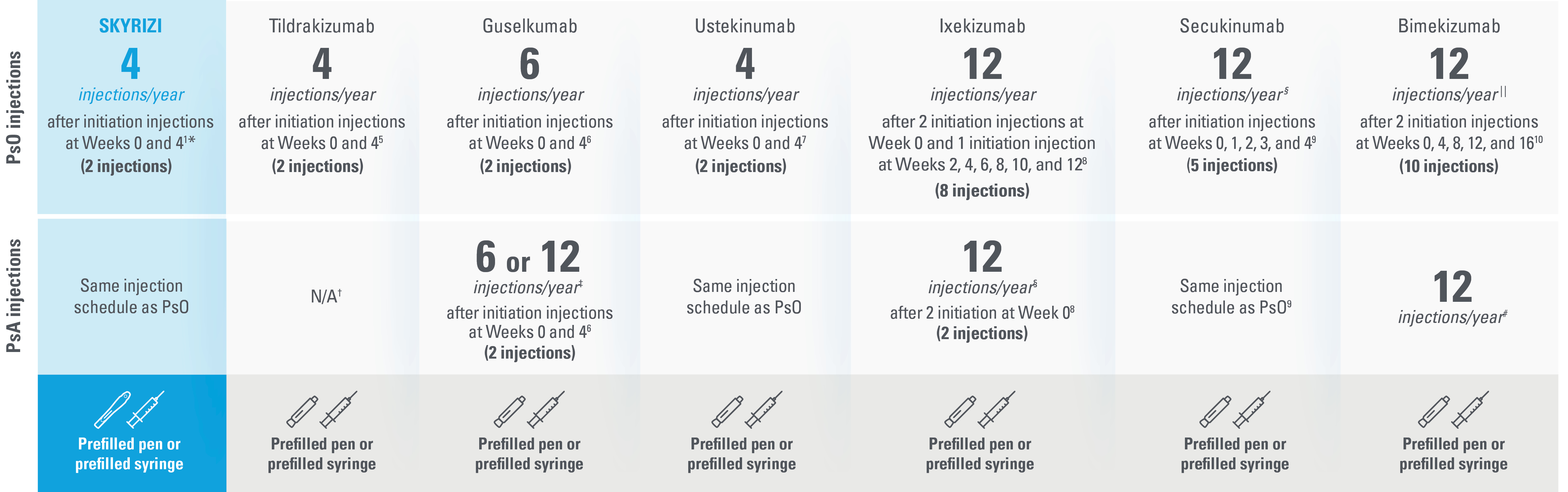
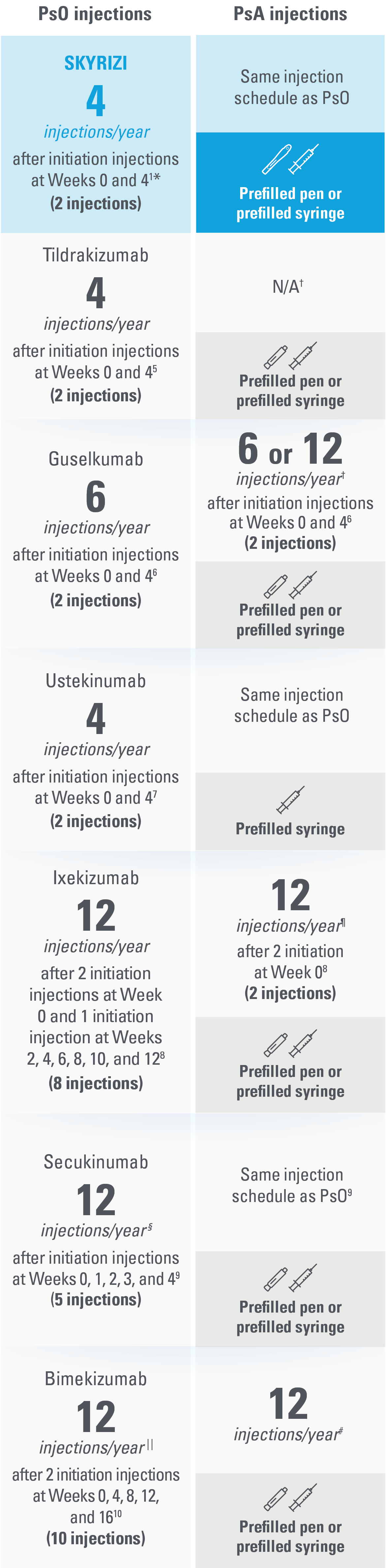
SKYRIZI: The ONLY treatment for adults with PsO and/or PsA with just 4 injections per year* with a prefilled pen and the freedom to choose a pen or syringe1,5-10
This presentation is not intended to compare the efficacy or safety of the treatments shown. While these factors are important, there are additional considerations for selecting a treatment. Please refer to each productʼs Summary of Product Characteristics for additional information.
The table above reflects devices available as of June 2023.
| * | Maintenance dosing (one 150-mg subcutaneous injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. |
| † | Not indicated for psoriatic arthritis as of June 2023.5,10 |
| ‡ | For PsA patients at high risk for joint damage according to clinical judgment, a dose of 100 mg every 4 weeks may be considered, instead of every 8 weeks.6 |
| § | The recommended dose is 160 mg by subcutaneous injection (two 80-mg injections) at Week 0, followed by 80 mg (one injection) every 4 weeks thereafter. For PsA patients with concomitant moderate to severe PsO, the recommended dosing regimen is the same as for PsO.8 |
| II | Patients with PsA with concomitant moderate to severe PsO or who are anti-TNFα inadequate responders, a 300-mg dose is recommended. Dose adjustment may be needed in certain patients depending on clinical response.9 |
| ¶ | For some patients with a body weight of ≥120 kg who did not achieve complete skin clearance at Week 16, dose recommendation is 2 injections every 4 weeks after Week 16 instead of every 8 weeks.10 |
| # | For PsA patients with coexistent moderate to severe PsO, the recommended dose is the same as for PsO [320 mg (given as 2 subcutaneous injections of 160 mg each) at Week 0, 4, 8, 12, 16 and every 8 weeks thereafter]. |
SKYRIZI is intended for use under the guidance of a healthcare professional. Patients may self-inject SKYRIZI after training in subcutaneous injection technique. Instruct patients to inject the full 150-mg dose and to read the Instructions for Use before administration.
Find out more about SKYRIZI
References
- SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; February 2023.
- Leonardi C, Gordon K, Longcore M, Gu Y, Puig L. Weight-based analysis of psoriasis area and severity index improvement at 52 weeks of risankizumab or ustekinumab treatment: an integrated analysis of patients with moderate-to-severe plaque psoriasis. Presented at: 24th World Congress of Dermatology (WCD); June 10–15, 2019; Milan, Italy. Poster 5248.
- Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830-2838. doi:10.1111/jdv.16521
- Blauvelt A, Gordon KB, Lee P, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. J Dermatolog Treat. 2022:33(4):2085-2093. doi:10.1080/09546634.2021.1914812
- ILUMETRI [Summary of Product Characteristics]. Almirall S.A.; February 2023.
- TREMFYA [Summary of Product Characteristics]. Janssen-Cilag International NV; July 2022.
- STELARA [Summary of Product Characteristics]. Janssen-Cilag International NV; December 2022.
- TALTZ [Summary of Product Characteristics]. Eli Lilly Nederland B.V.; February 2023.
- COSENTYX [Summary of Product Characteristics]. Novartis Europharm Limited; January 2023.
- BIMZELX [Summary of Product Characteristics]. UCB Pharma S.A.; June 2023.