SKYRIZI: An IL-23/p19 inhibitor2-7
KEEPsAKE-1/2 PsA data: ACR20/50/70
DURABLE CONTROL OF PsA SIGNS AND SYMPTOMS
could mean everything for your patients
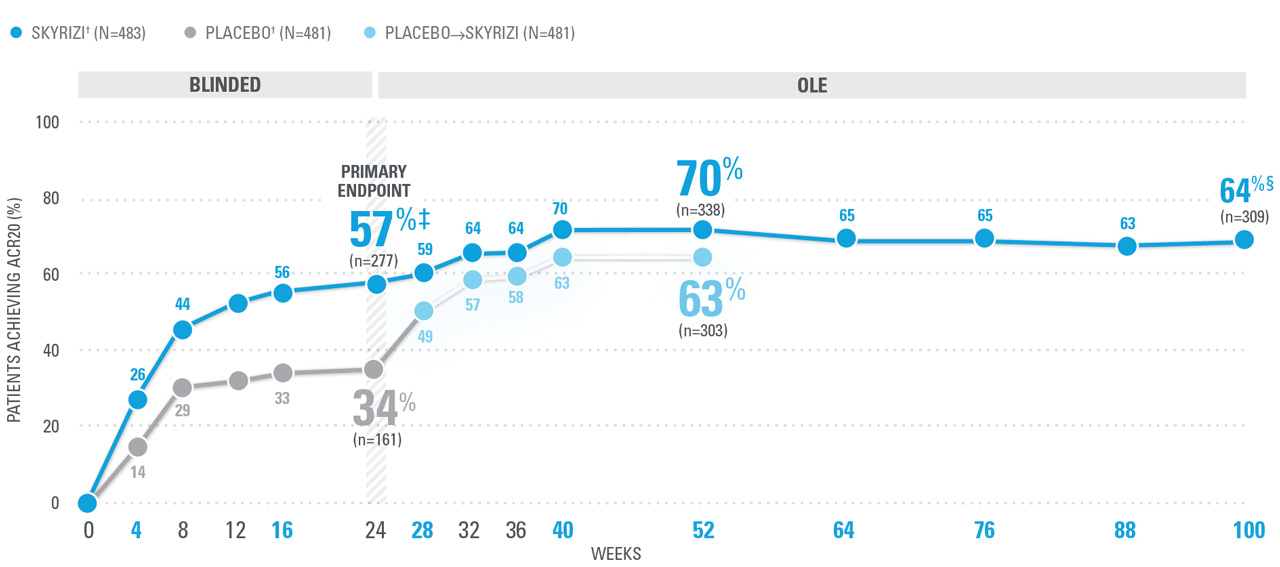
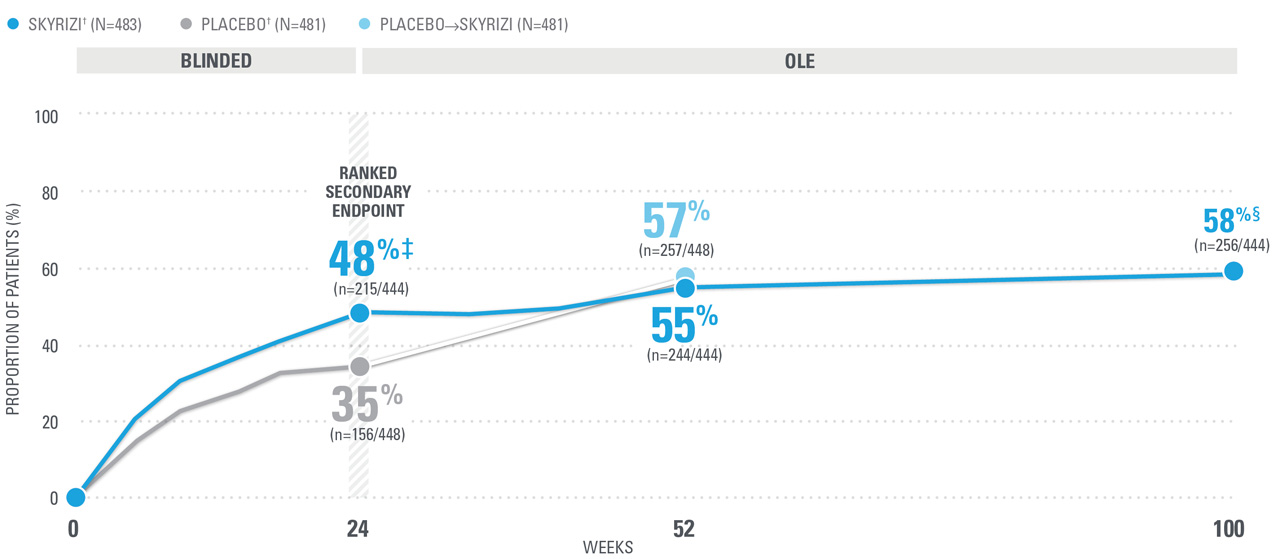
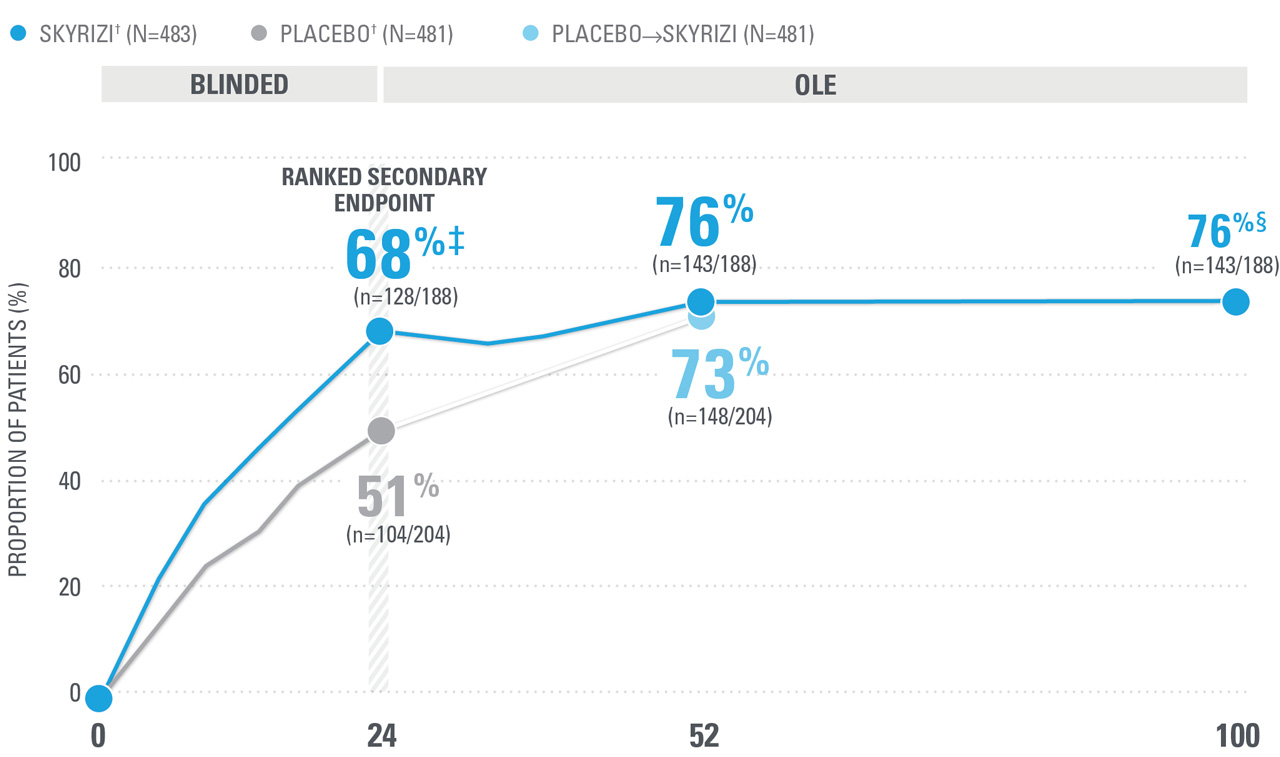
~7 OUT OF 10 PATIENTS ACHIEVED ACR20 AT WEEK 52 (NRI)1,8-10*
| SKYRIZI doses denoted in blue: For ACR 20/50/70, participants received 150 mg SKYRIZI at Week 0, Week 4, and every 12 weeks thereafter. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. | |
| * | 5ummarized from KEEPsAKE-1 (bio-naïve population). |
| † | 65.5% of subjects from KEEPsAKE-1 taking placebo and 65% of SKYRIZI patients were receiving concomitant MTX. 10.2% of patients taking placebo and 10.8% of SKYRIZI patients were receiving concomitant nonbiologic DMARDs other than MTX.8 |
| ‡ | P≤0.001 vs placebo.1 |
| § | Weeks 52-100 were NRI-C and NRI-MI (see additional study details). |
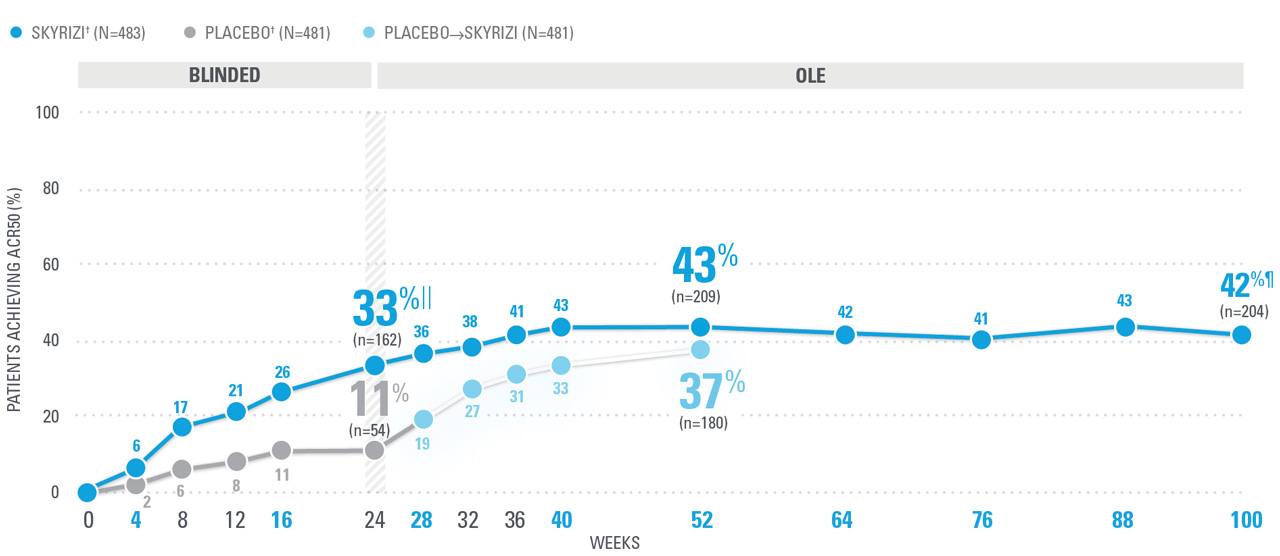
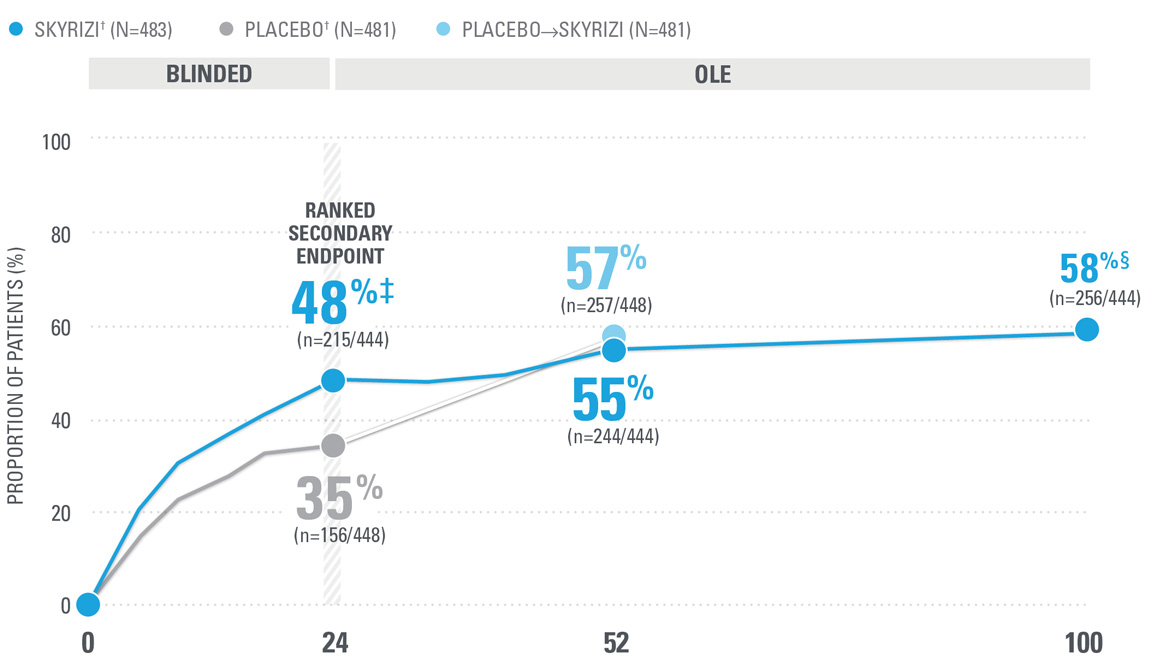
OVER 40% OF SKYRIZI PATIENTS ACHIEVED ACR50 AT WEEK 52 WITH CONSISTENT RATES THROUGH WEEK 100 (NRI)1,8-10*
| ACR50 was a prespecified, non-ranked secondary endpoint. | |
| || | Nominal P≤0.001 vs placebo, not multiplicity-controlled.1 |
| ¶ | Weeks 52-100 were NRI-C and NRI-MI (see additional study details). |
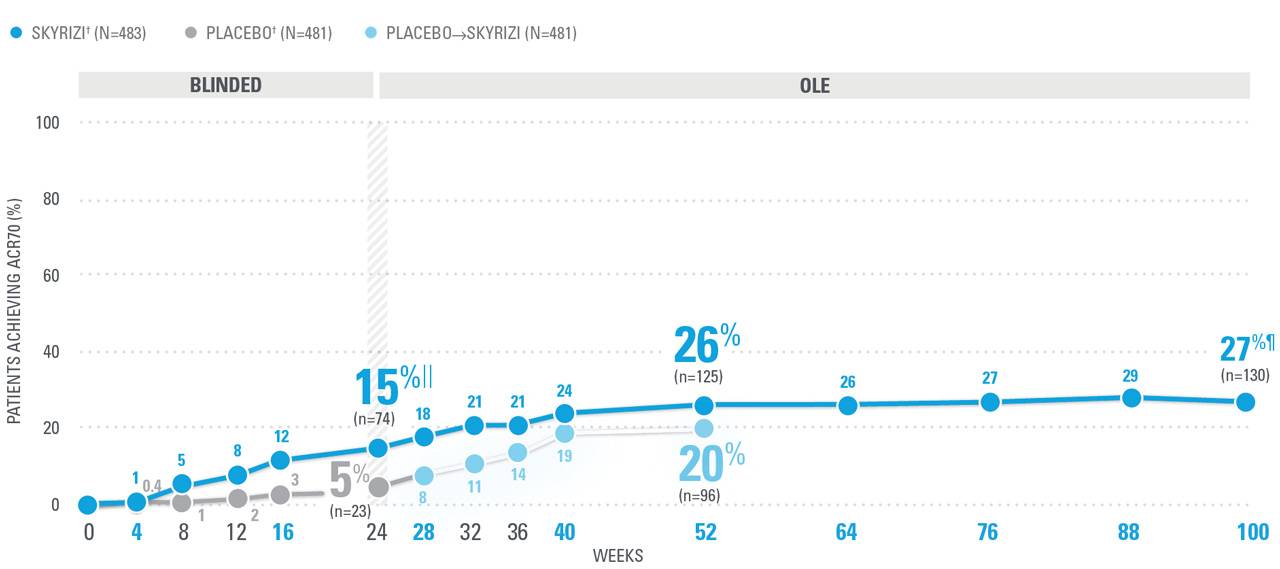
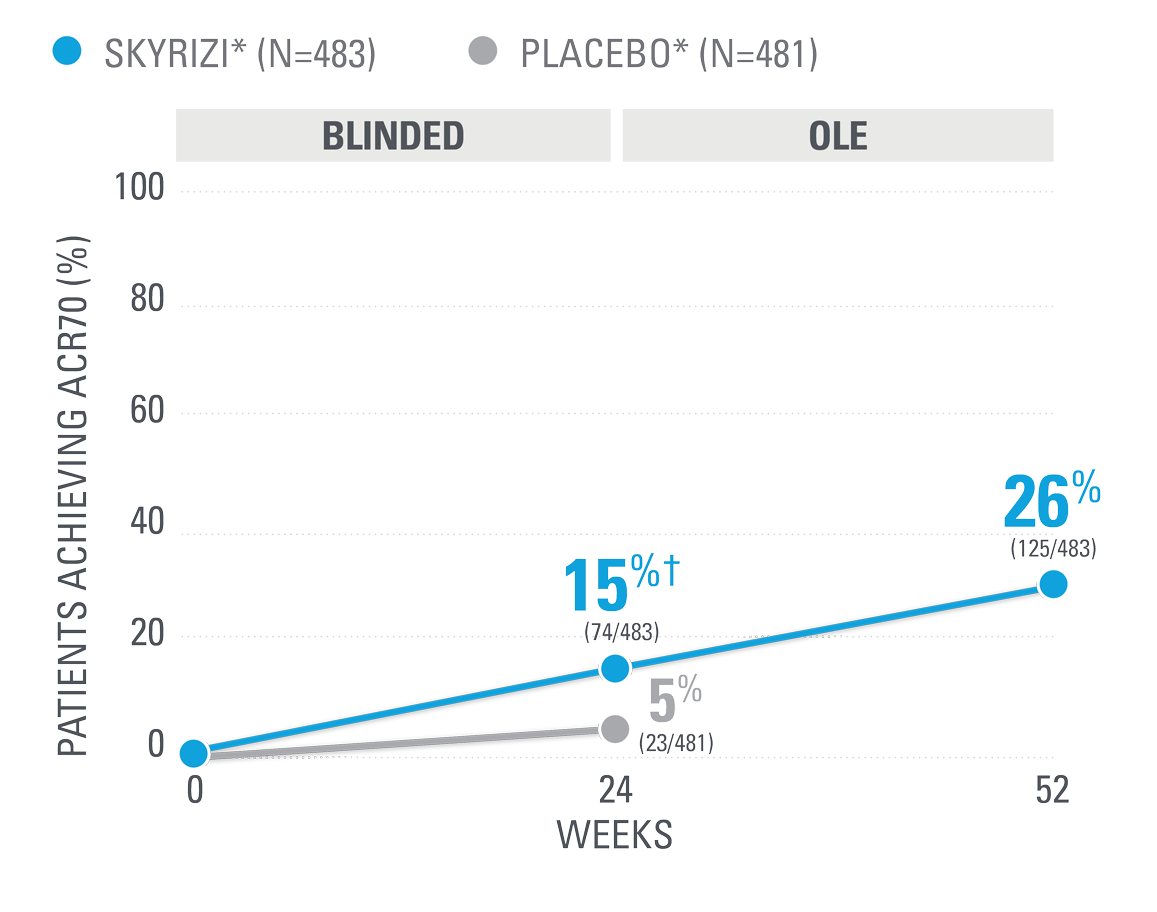
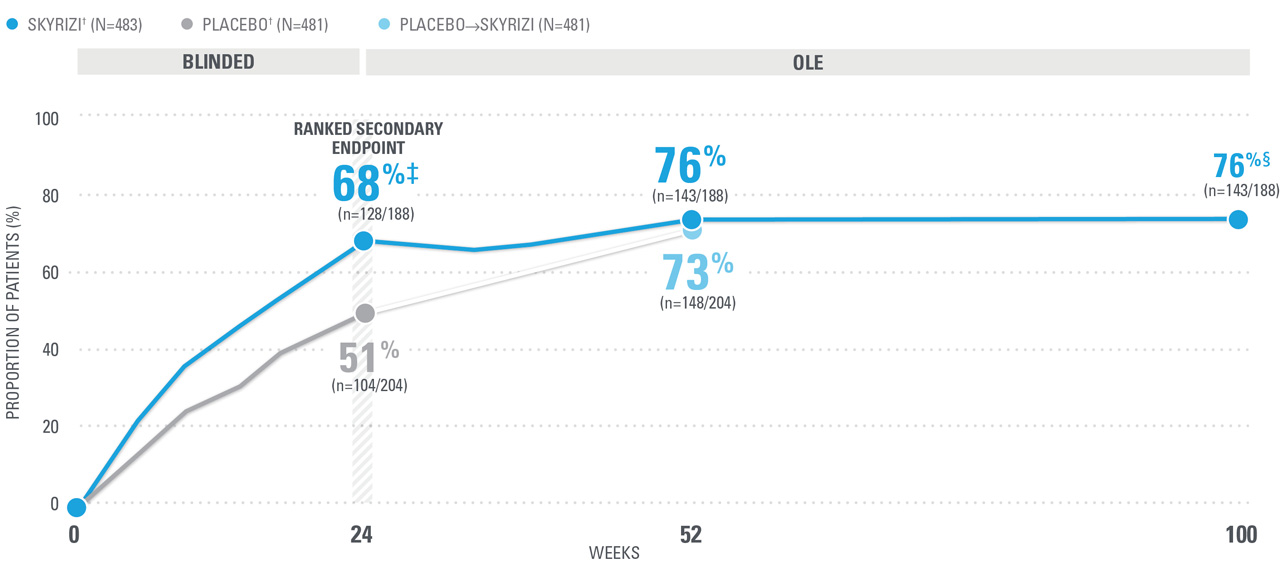
ACR70 RESPONSE RATES WITH SKYRIZI THROUGH WEEK 100 (NRI)1,8-10*
| ACR70 was a prespecified, non-ranked secondary endpoint. | |
| || | Nominal P≤0.001 vs placebo, not multiplicity-controlled.1 |
| ¶ | Weeks 52-100 were NRI-C and NRI-MI (see additional study details). |
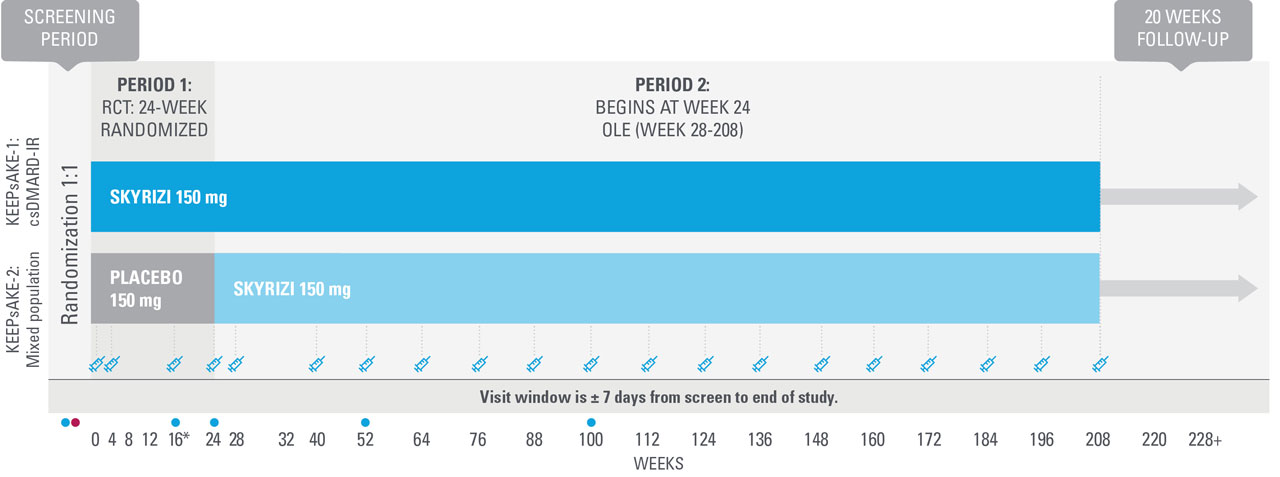
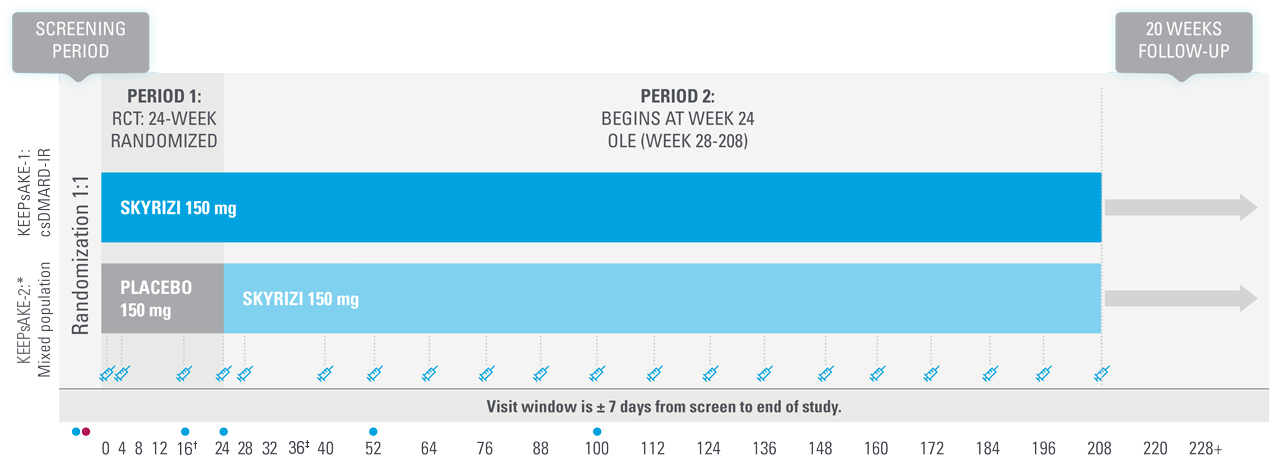
SKYRIZI is dosed 150 mg at Week 0, Week 4, and every 12 weeks thereafter.
Bilateral radiographs of hand and feet: KEEPsAKE-1. KEEPsAKE-2.
Mixed population=50% csDMARD-IR, 50% Bio-IR population.
| * | At Week 16, subjects classified as nonresponders (defined as not achieving at least a 20% improvement in either or both tender joint count and swollen joint count at both Week 12 and Week 16 compared to baseline) had the option to add or modify rescue concomitant medications/therapy. |
| † | Starting at Week 36, subjects classified as nonresponders were discontinued from study drug. |
KEEPsAKE-1/2 PsA data: Enthesitis/Dactylitis
DURABLE, COMPLETE RESOLUTION OF ENTHESITIS AND DACTYLITIS could mean everything for your patients
CONSISTENT RATES OF COMPLETE RESOLUTION OF ENTHESITIS WITH SKYRIZI (NRI)1,8-10*
CONSISTENT RATES OF COMPLETE RESOLUTION OF DACTYLITIS WITH SKYRIZI (NRI)1,8-10||
| SKYRIZI was dosed 150 mg (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. | ||
| * | Defined as LEI=0 among patients with LEI>0 at baseline. Pooled data from KEEPsAKE-1 and KEEPsAKE-2.1 | |
| † | 59.6% of subjects from both KEEPsAKE-1 and KEEPsAKE-2 studies were receiving concomitant MTX, 11.6% were receiving concomitant nonbiologic DMARDs other than MTX, and 28.9% were receiving SKYRIZI monotherapy.1 | |
| ‡ | P≤0.001.1 | |
| § | Continuous data points (Week 100) are reported by mixed-effect model repeated measurement (MMRM) and categorical endpoints are reported by as observed with missing data imputed as nonresponders except those missing due to COVID-19, which are imputed by multiple imputation (NRI-MI). | |
| || | Defined as LDI=0 among patients with LDI>0 at baseline. Pooled data from KEEPsAKE-1 and KEEPsAKE-2.1 |
NRI=nonresponder imputation; OLE=open-label extension.
KEEPsAKE-1 and KEEPsAKE-2: study design1,8,11
Two randomized, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA.
SKYRIZI is dosed 150 mg at Week 0, Week 4, and every 12 weeks thereafter.
Bilateral radiographs of hand and feet: KEEPsAKE-1. KEEPsAKE-2.
| * | Mixed population=50% csDMARD-IR, 50% Bio-IR population. |
| † | At Week 16, subjects classified as nonresponders (defined as not achieving at least a 20% improvement in either or both tender joint count and swollen joint count at both Week 12 and Week 16 compared to baseline) had the option to add or modify rescue concomitant medications/therapy. |
| ‡ | Starting at Week 36, subjects classified as nonresponders were discontinued from study drug. |
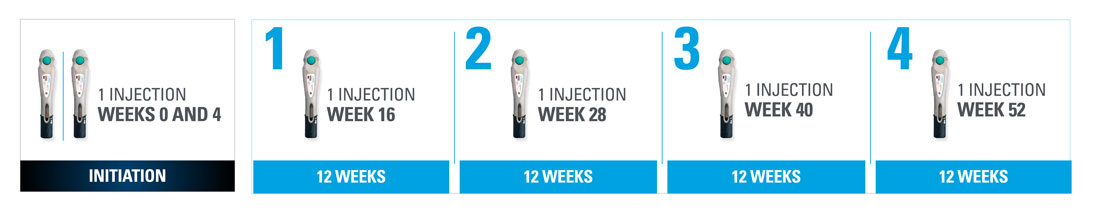
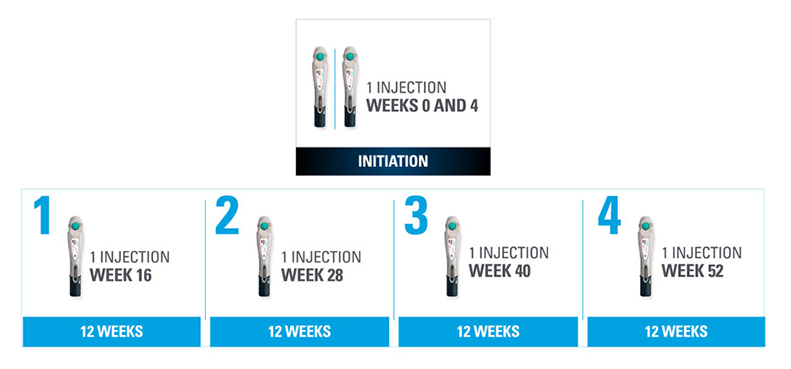
Nothing more than 4 INJECTIONS PER YEAR after initiation doses for both PsO and PsA patients1*
NO DOSE ADJUSTMENT regardless of baseline characteristics, including BMI and weight1,12,13†
- SKYRIZI is dosed 150 mg (one 150-mg subcutaneous injection) at Week 0, Week 4, and every 12 weeks thereafter.1
- Low rate of injection site reactions at Week 16 (based on an analysis of 5 PsO clinical trials: SKYRIZI 1.5% vs placebo 1.0%)14
| — | In a long-term analysis (up to 7.8 years), rates of injection site reactions were low for SKYRIZI PsO patients (2.9 E/100 PY).15‡ |
Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks.
| * | Maintenance dosing (1 injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. If a dose is missed, the dose should be administered as soon as possible. Thereafter, dosing should be resumed at the regular scheduled time. |
| † | Risankizumab clearance and volume of distribution increase as body weight increases, which may result in reduced efficacy in subjects with high body weight (>130 kg). However, this observation is based on a limited number of subjects. |
| ‡ | Week 16 (5-study pool) and long-term analysis (median duration of treatment 4.2 years [ranging from 3 days to 7.8 years], 20-study pool) represent different pools of patients with varying lengths of treatment exposure included in the long-term data set.15 |
SKYRIZI one injection per dose:
SAME EFFICACY AND SAFETY PROFILE
Same active ingredient | Demonstrated bioequivalence
NOW EVEN SIMPLER WITH
SKYRIZI 150 mg bioequivalence data1
Bioequivalence was demonstrated between a single SKYRIZI 150-mg injection and two SKYRIZI 75-mg injections in a prefilled syringe. Bioeqivalence was also demonstrated between SKYRIZI 150 mg in a prefilled syringe and a prefilled pen.
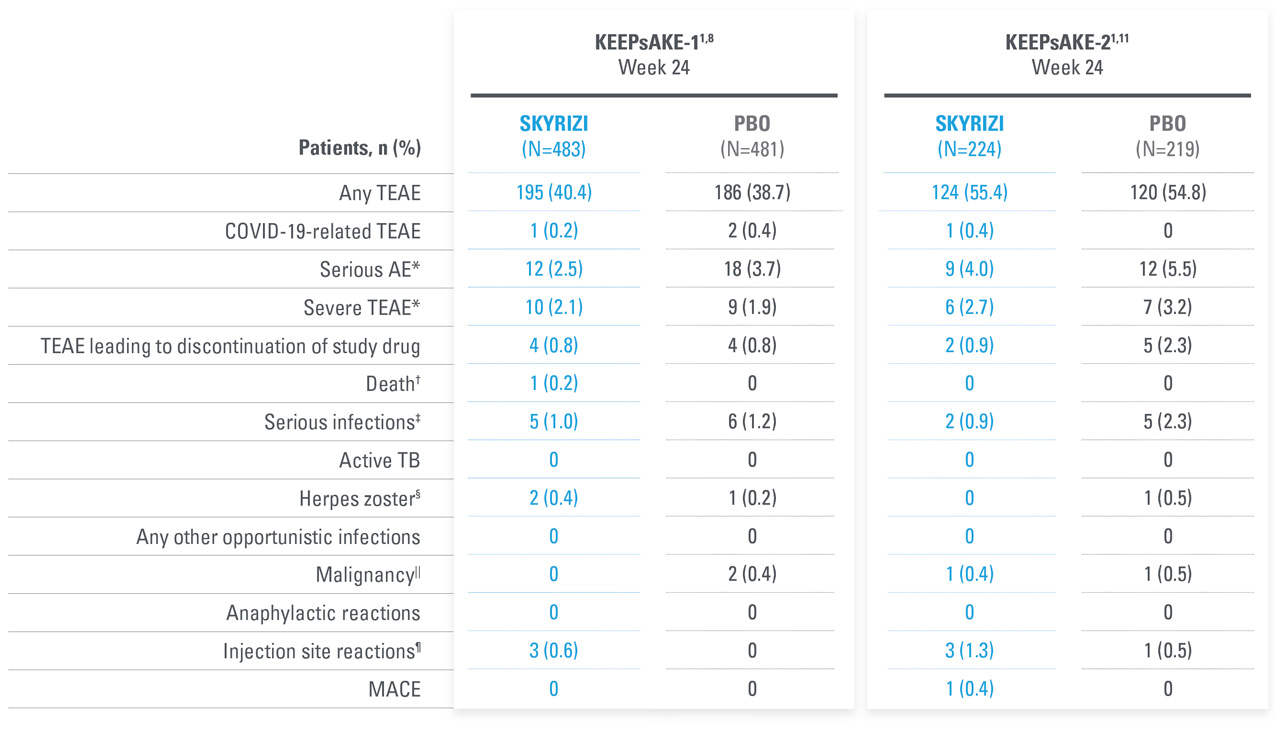
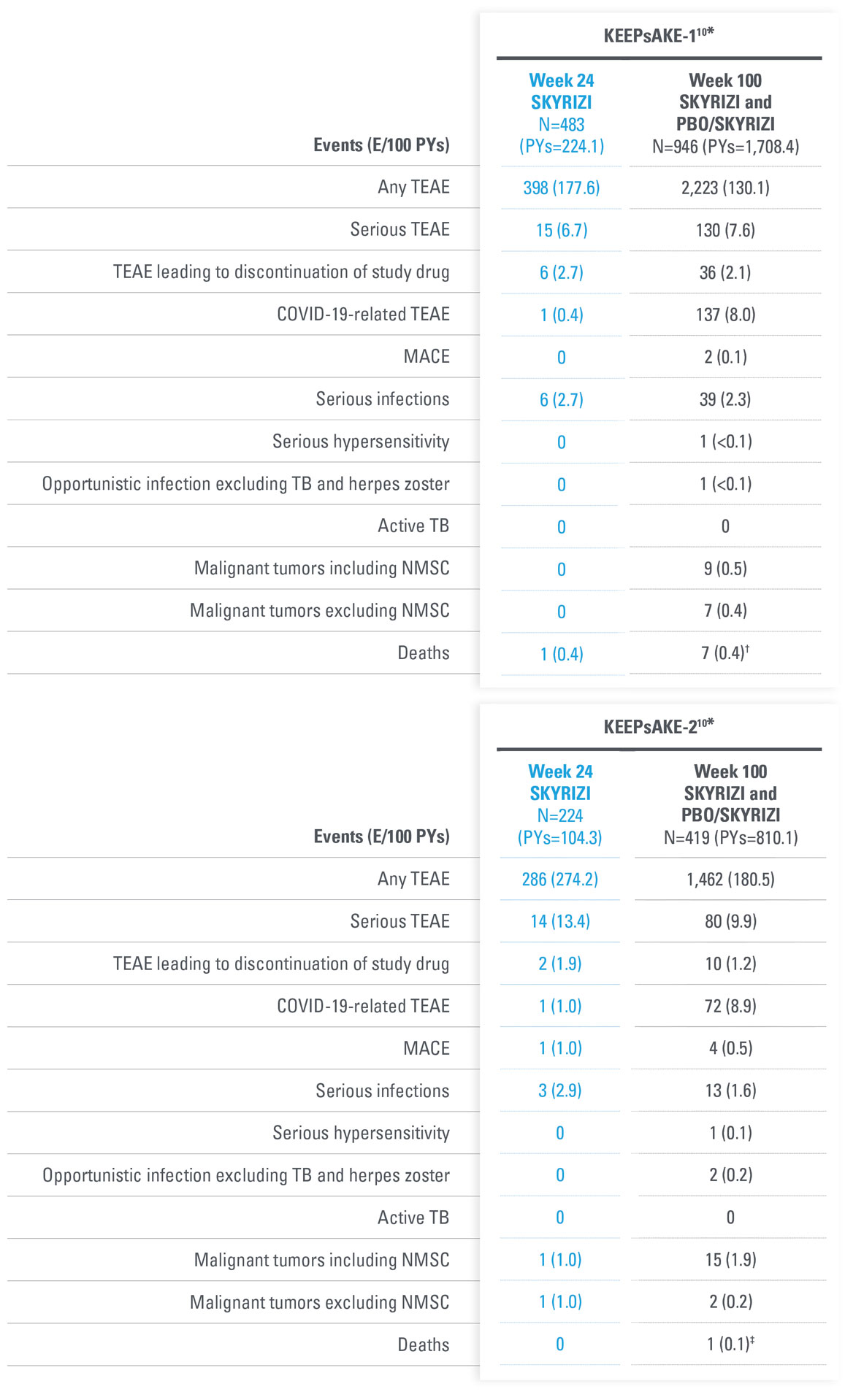
| * | KEEPsAKE-1: Except for pneumonia, which was reported for 2 patients (0.4%) in the placebo group, no serious AE or severe TEAE was reported for >1 patient in either group. |
| † | One death (urosepsis) in an 81-year-old male patient. |
| ‡ | KEEPsAKE-1 SKYRIZI: Urosepsis (1 patient, resulting in death), cellulitis (1 patient), gastroenteritis (1 patient), COVID-19 pneumonia (1 patient), and viral upper respiratory tract infection leading to pneumonia (1 patient); placebo: pneumonia (2 patients), oral bacterial infection (one patient), dysentery (1 patient), appendicitis (1 patient), and cellulitis (1 patient). KEEPsAKE-2 SKYRIZI: abscess and cellulitis (1 patient) and gastroenteritis (1 patient); placebo: erysipelas, gastroenteritis, postoperative abscess, upper respiratory tract infection, and urinary tract infection (each reported for 1 patient). |
| § | KEEPsAKE-1: All nonserious, resolved with oral antiviral agents and did not result in discontinuation of the study drug. |
| || | KEEPsAKE-2: Mild, Both were nonmelanoma skin cancer. |
| ¶ | All nonserious and did not result in discontinuation of the study drug. |
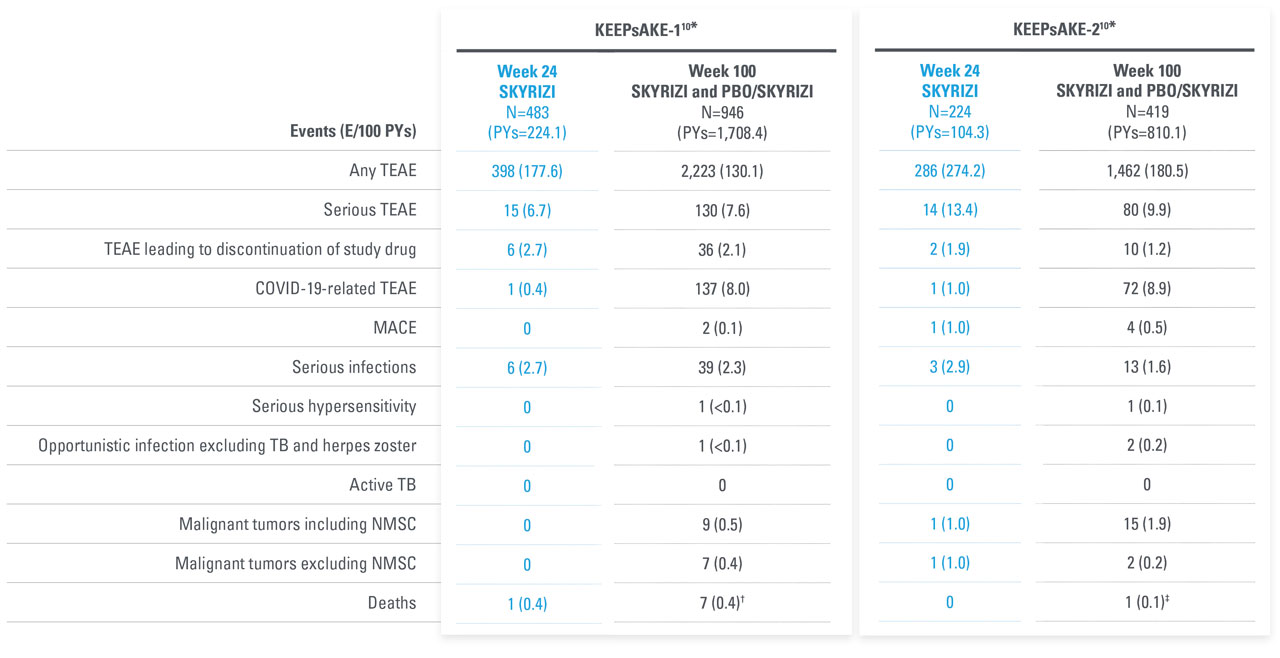
AEs THROUGH WEEK 100 IN PsA DURING ADMNISTRATION OF SKYRIZI
No new safety signals observed
| SKYRIZI dosed 150 mg at Week 0, Week 4, and every 12 weeks thereafter. | |
| * | Data includes all patients who received SKYRIZI 150 mg, including those who started on SKYRIZI 150 mg at randomization and who switched from placebo to SKYRIZI 150 mg after Week 24. |
| † | 6 subjects with fatal treatment-emergent events. 2 deaths were related to COVID-19. 1 due to complications related to acute leukemia; 1 with anemia from diverticulosis died due multi-organ failure from septicemia as a complication from anastomosis surgery. 1 81-year-old patient with dementia was hospitalized for pneumonia, developed urosepsis, and complications resulted in death. 1 patient hospitalized for anxiety and depression developed septicemia, nausea, vomiting, fever, and loss of appetite 1 week after discharge and then died from from an unknown cause another week later. Additionally, 1 subject died on Day 363 (166 days after last dose) due to cardiorespiratory arrest. |
| ‡ | 1 death with the event of coronary artery plaque rupture. Subject had multiple risk factors such as obesity, long history of smoking, hypertension, hypercholesterolemia, and a family history of cardiovascular disease. |
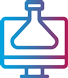
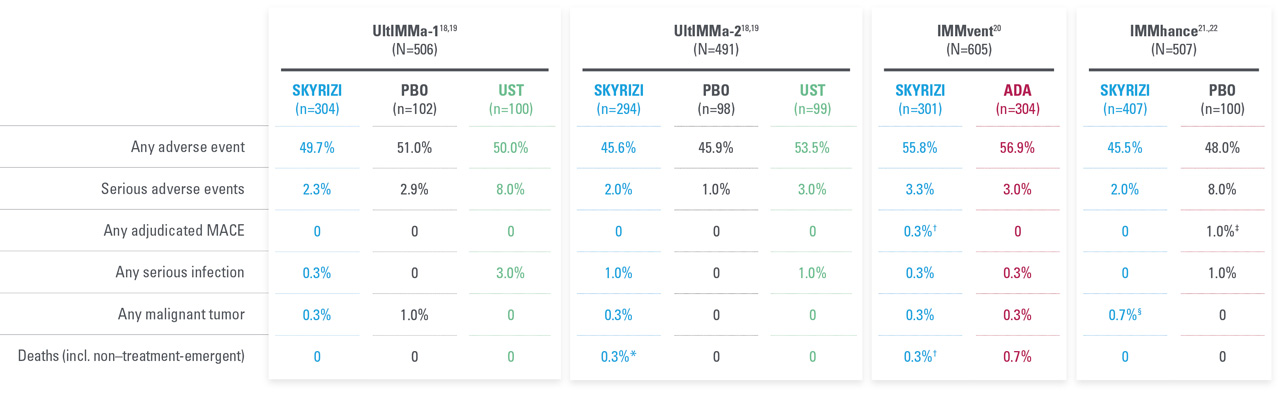
A FAVORABLE PsO SAFETY PROFILE1
No cases of active TB reported in controlled periods of PsO and PsA trials, inlcuding 31 IMMhance study patients with latent TB who did not receive prophylaxis1,18,20-22
Prior to and during SKYRIZI
treatment, evaluate and monitor
patients for TB. Consider anti-TB
therapy prior to initiating
SKYRIZI in patients with
history of latent or active TB.1
Safety profile in PsA consistent with safety profile observed in PsO1
| * | Integrated all-risankizumab safety data set from 20 completed or ongoing Phase 1–4 risankizumab clinical trials in plaque psoriasis (data cutoff March 25, 2022). Median duration of treatment was 4.2 years (ranging from 3 days to 7.8 years). |
Important contextual information1
SKRYIZI is contraindicated in patients with clinically important active infections (e.g. active tuberculosis).
Tuberculosis: Prior to initiating treatment with SKYRIZI, patients should be evaluated for tuberculosis (TB) infection. Patients receiving SKYRIZI should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating SKYRIZI in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Lab monitoring: SKYRIZI may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, SKYRIZI should be used with caution.
Treatment with SKYRIZI should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated. Patients treated with SKYRIZI should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and SKYRIZI should not be administered until the infection resolves.
| * | UltIMMa: One non–treatment-emergent death of unknown cause on study Day 189 that occurred 161 days after the last dose of study drug. |
| † | IMMvent: One patient with acute myocardial infarction on study Day 73 (event was not considered to be study drug related by investigator). |
| ‡ | IMMhance: One patient with stroke reported as ischemic stroke on study Day 95. |
| § | IMMhance: One patient with esophageal carcinoma reported on study Day 16, with patient experiencing 40 lbs weight loss six months prior to study participation; one patient with malignant melanoma in situ reported on study Day 102, study drug was not interrupted; one patient with a cutaneous squamous cell carcinoma reported on study Day 89, study drug not interrupted. |
A safety profile similar to ustekinumab in PsO through Week 52 during RCTs:18
- UltIMMa-1—Any AE: SKYRIZI 61.3% (n=182/297) vs ustekinumab 66.7% (n=66/99); Serious AEs: SKYRIZI 5.4% (n=16/297) vs ustekinumab 4.0% (n=4/99); Infections: SKYRIZI 37.7% (n=112/297) vs ustekinumab 41.4% (n=41/99)
- UltIMMa-2—Any AE: SKYRIZI 55.7% (n=162/291) vs ustekinumab 74.5% (n=70/94); Serious AEs: SKYRIZI 4.5% (n=13/291) vs ustekinumab 4.3% (n=4/94); Infections: SKYRIZI 34.7% (n=101/291) vs ustekinumab 48.9% (n=46/94)
Through Week 52, the frequency of the adverse reactions was similar to the safety profile observed during the first 16 weeks of treatment. Through Week 52, the exposure-adjusted rates of serious adverse events per 100 subject-years were 9.4 for subjects treated with SKYRIZI and 10.9 for those treated with ustekinumab. For those subjects exposed to a maximum of 77 weeks of SKYRIZI, no new adverse reactions were identified compared to the first 16 weeks of treatment.1
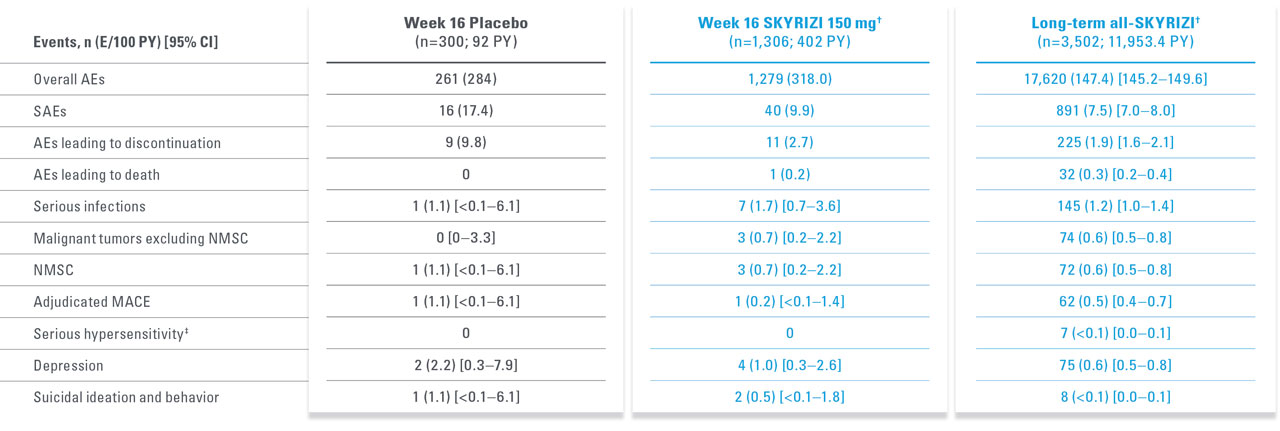
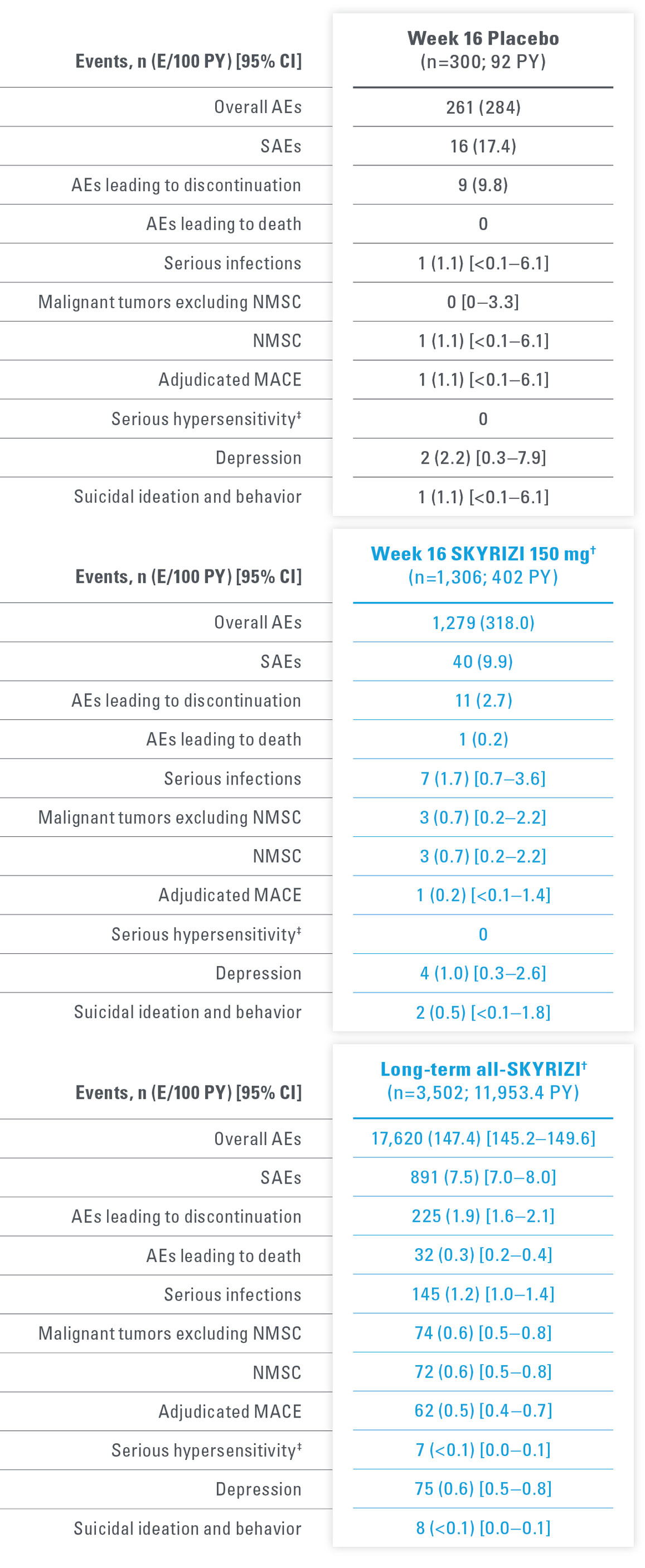
LONG-TERM PsO SAFETY PROFILE CONSISTENT UP TO 7.8 YEARS15,17*
Adverse events of special interest in a pooled analysis from the first 16 weeks of treatment up to 7.8 years
SKYRIZI warnings and precautions include infections, tuberculosis, and immunizations.1
- Most commonly reported serious infections were sepsis and pneumonia.
- Most common infectious AEs were nasopharyngitis and upper respiratory tract infection (overall infections over long term: 45.9 events per 100 patient-years).
- One case of active TB; patient had latent TB diagnosed at screening and the nonserious event occurred 4 years after study initiation (rate over long term: <0.1 events per 100 patient-years).
- No cases of systemic candidiasis in the long- or short-term analysis.
- Rate of MACE over the long term (0.5 E/100 PY) consistent with reference rates for MACE reported in PSOLAR.
- No cases of anaphylaxis reported.
- One case of IBD reported (worsening of ulcerative colitis in patient with history of the disease).
- A total of 32 (30 treatment-emergent) deaths were reported. Using data from the WHO (2015) the SMR for treatment-emergent deaths was 0.40 (0.27-0.58).
| * | Short-term safety through Week 16 was evaluated using data integrated from 5 Phase 2 and 3 trials in patients with moderate to severe plaque psoriasis: Trial 1311.2, UltIMMa-1, UltIMMa-2, IMMhance, and IMMvent. Long-term (median duration of treatment was 4.2 years [ranging from 3 days to 7.8 years]) safety was evaluated in a larger all-risankizumab data set from 20 Phase 1–3 completed and ongoing trials as of March 25, 2022. |
| † | Week 16 (5-study pool) and long-term (up to 7.8 years, 20-study pool) represent different pools of patients with varying lengths of treatment exposure included in the long-term set. Events counted in the Week 16 data are also included in the long-term data. |
| ‡ | Eczema (n=2), Stevens-Johnson syndrome (n=2), angioedema (n=1), drug hypersensitivity (n=1), and erythema multiforme (n=1). |