This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
Overactive bladder (OAB) can be debilitating and negatively affect quality of life6
OAB symptoms can be very distressing and embarrassing for patients, as well as disrupting normal daily functioning and reducing independence.6
A large proportion of OAB patients may remain untreated7
Of 1,916 men and women aged 40-74 years with bothersome OAB symptoms, 60% had spoken to a doctor, but only 27% of these (16% of the total cohort) were currently on medication.7
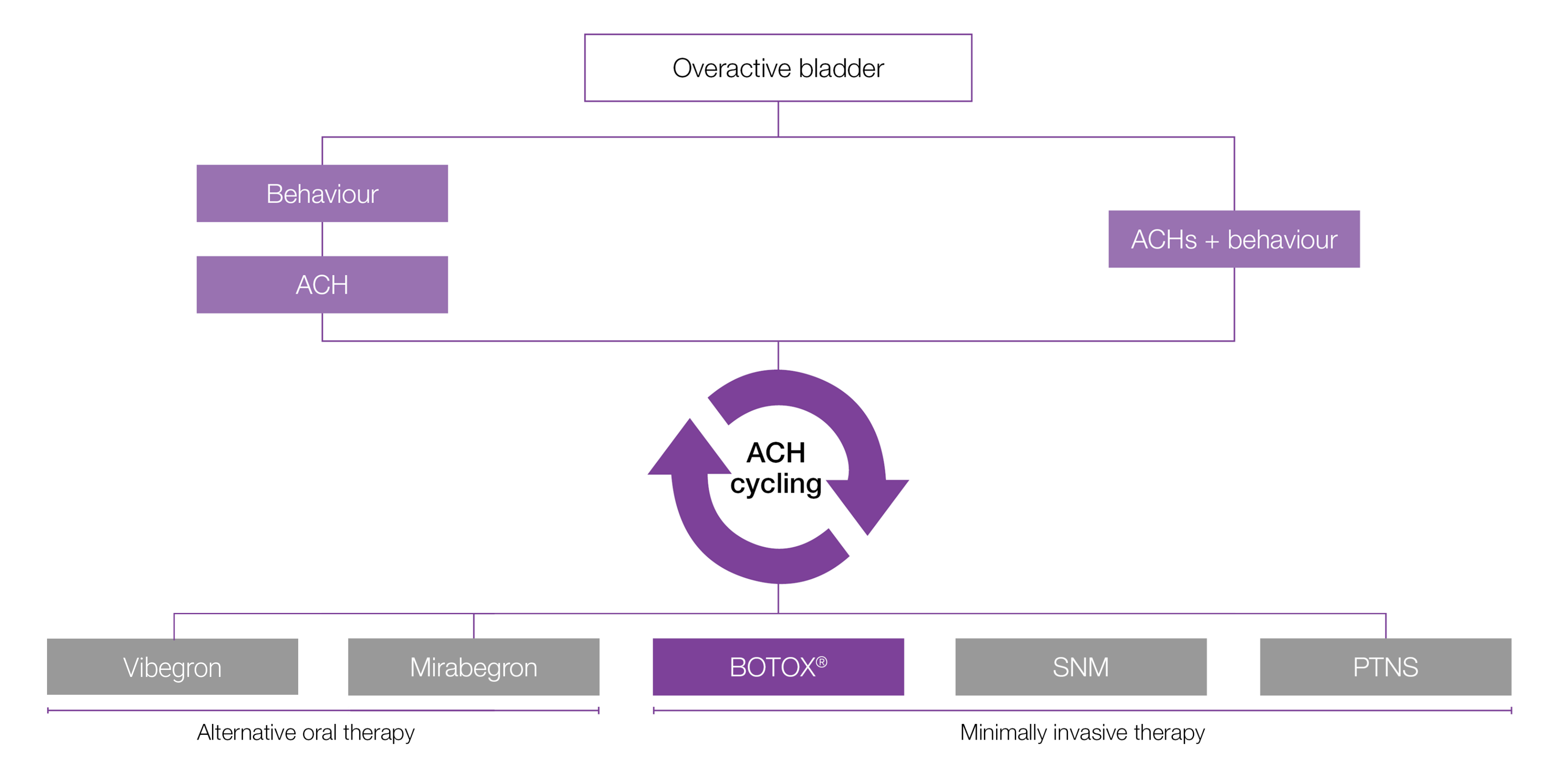
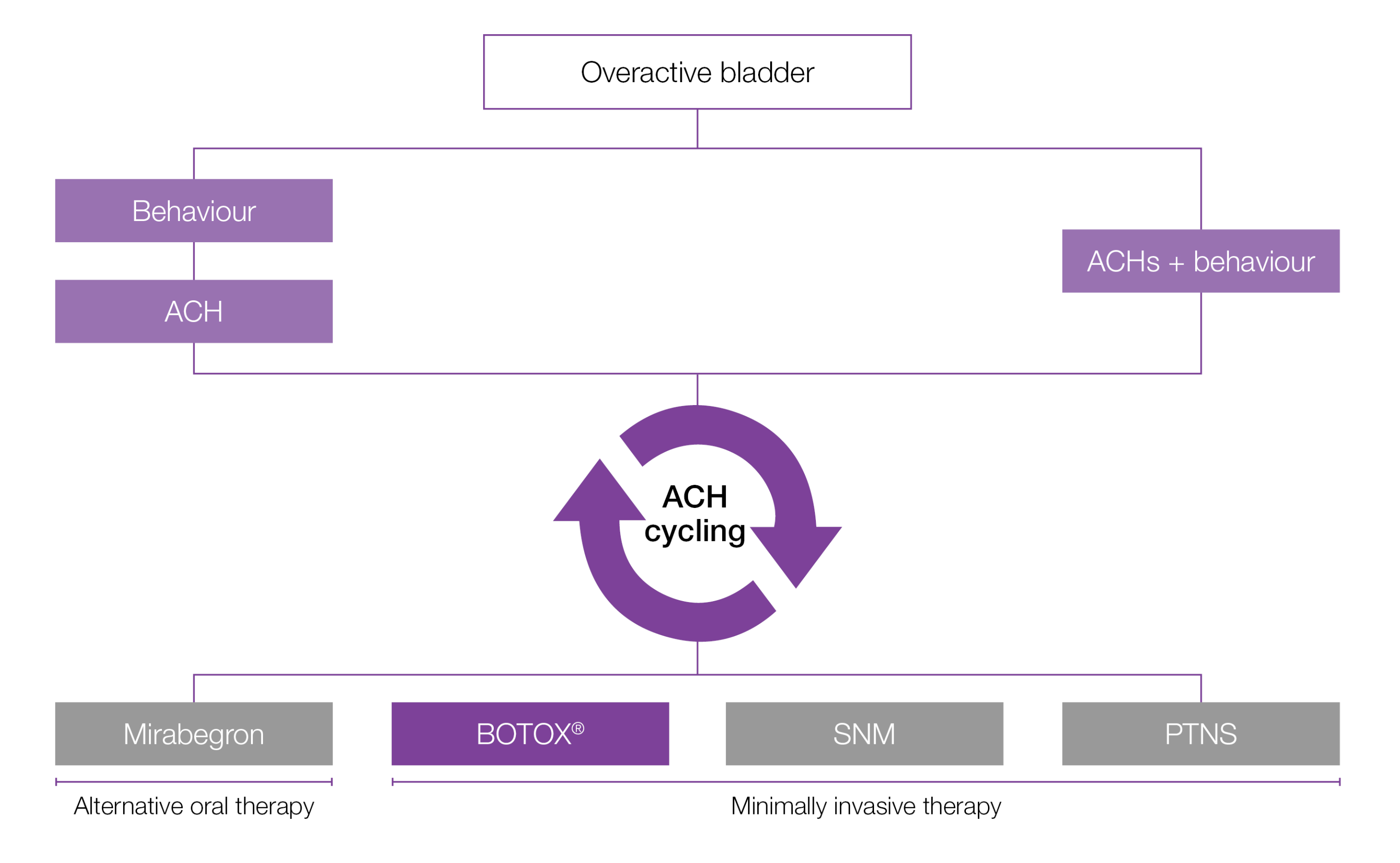
Are oral therapies always the right answer for OAB patients?
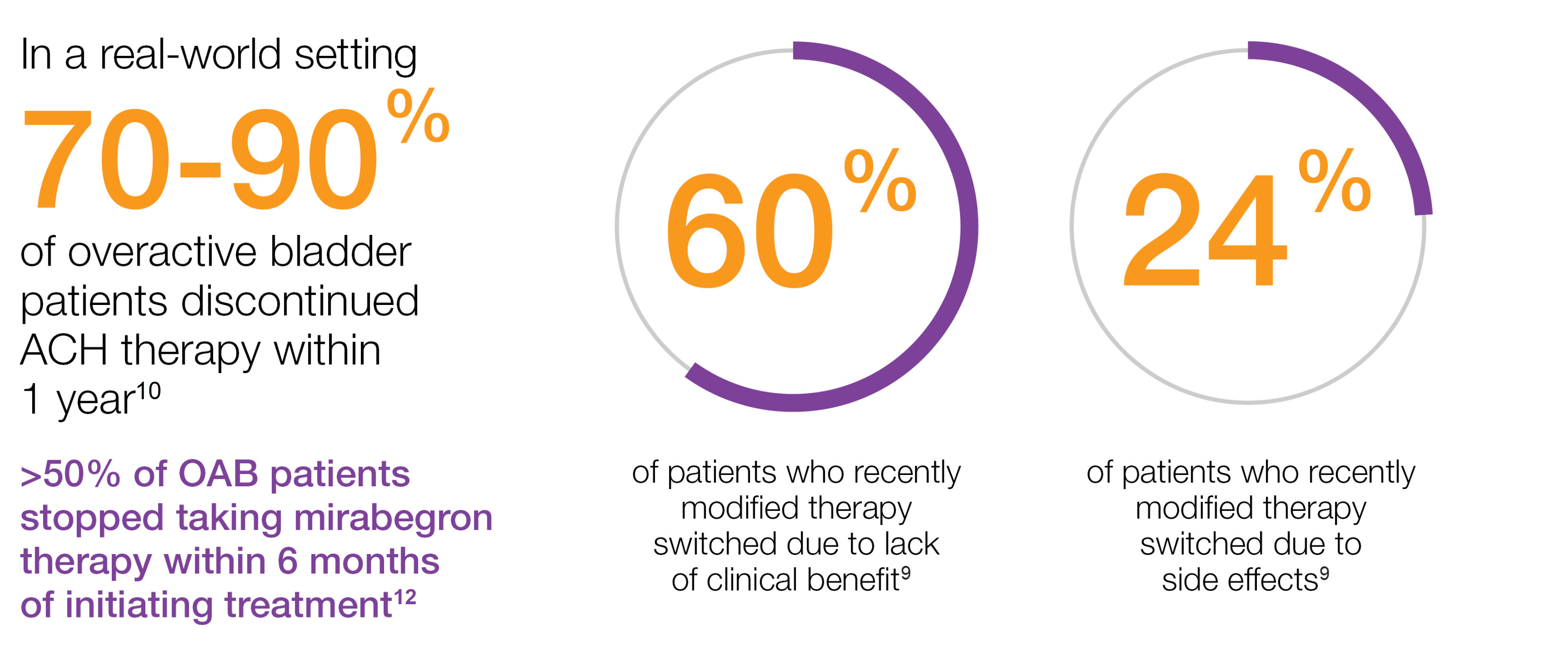
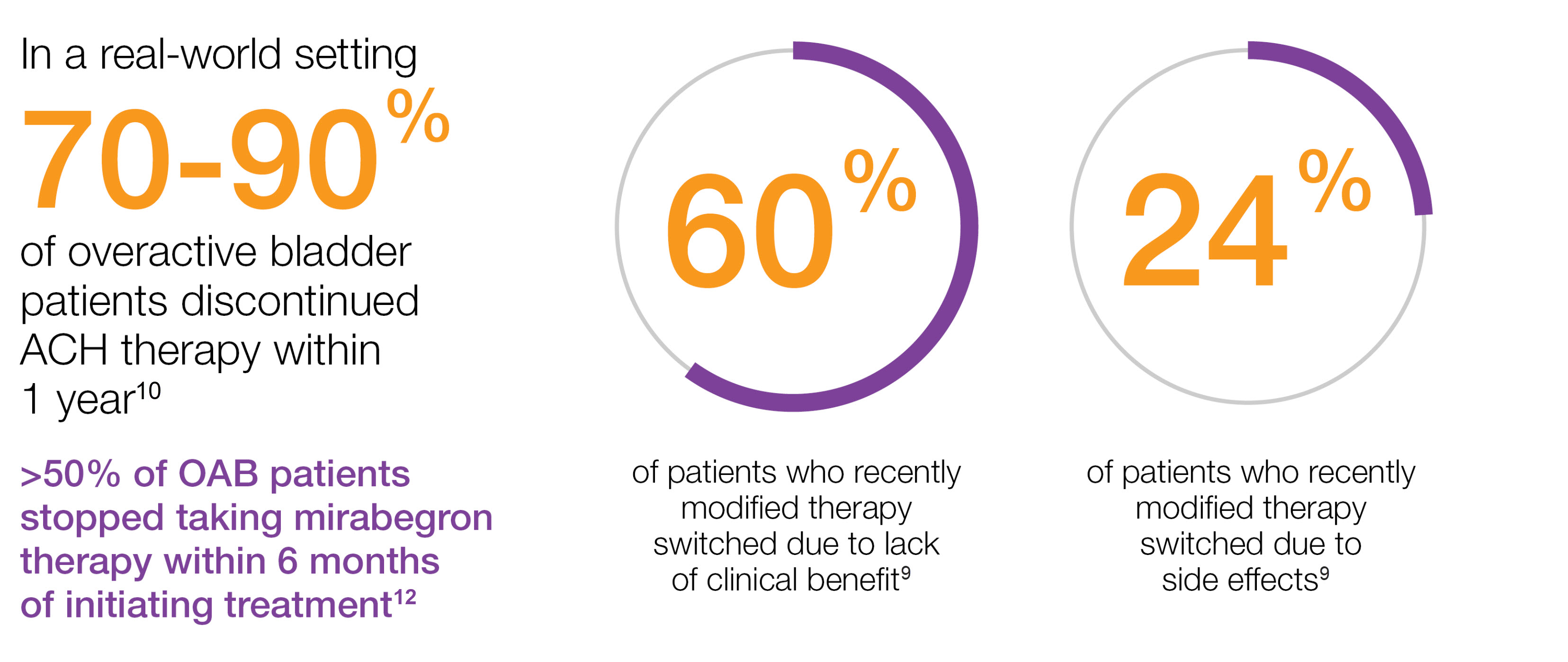
The majority of overactive bladder patients DISCONTINUED oral therapies10-12
Study context: An epidemiological, cross-sectional, non-interventional study of 2,038 evaluable OAB patients who had their treatment modified during the previous 3–4 months. The objective of this study was to identify the reasons why some patients do not respond and to look for reasons for changes in treatment and patient satisfaction with the new treatment.9
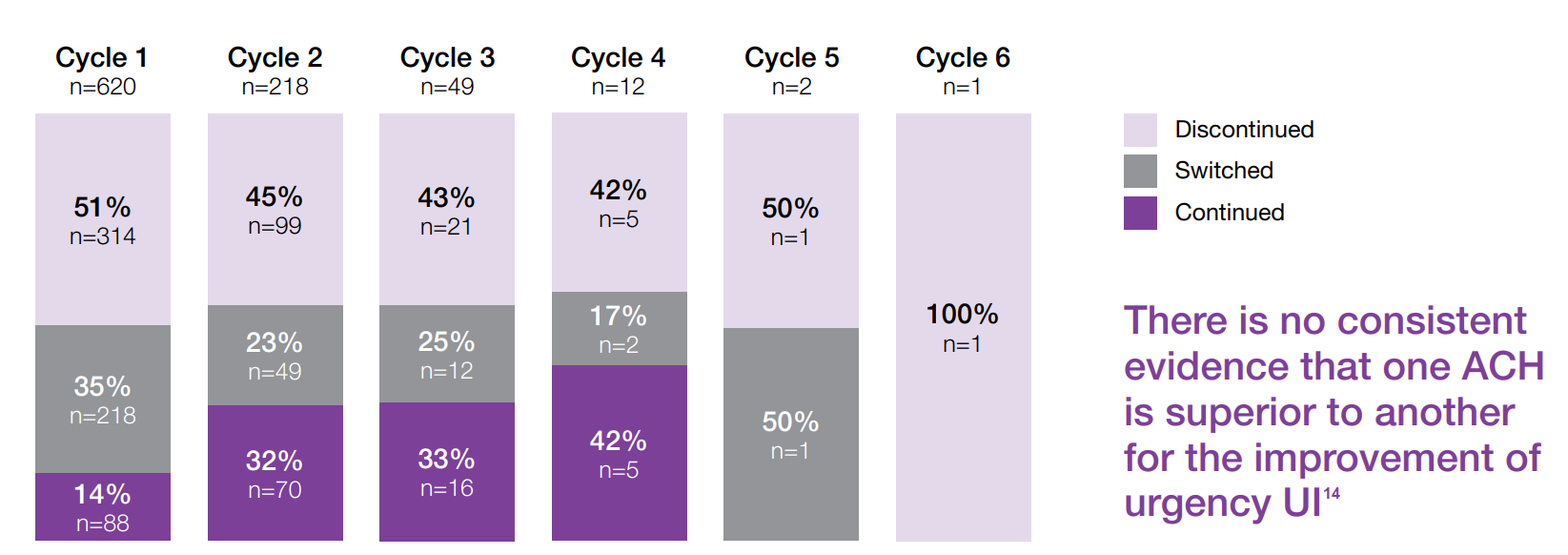
Anticholinergic cycling can be associated with high rates of treatment discontinuation13
620 patients initiated ACH therapy - 70% of patients DISCONTINUED by 3rd cycle13
Study context: Retrospective claims analysis from a California-based managed care organisation linked to a one-time patient survey of OAB patients with UI. The purpose was to describe treatment patterns and outcomes in wet-OAB patients with anticholinergics. A total of 620 patients were enrolled into the study – having met the following criteria: received ACH therapy between January 2008 and May 2012, had a diagnosis of OAB and reported having ≥1 UI episode per day at the time of the survey.13
Adapted from Chancellor M et al, 201613
- Of a cohort of 620 patients initiating ACH therapy, 71% had discontinued by the end of the study period (3 years).13
- ACHs are commonly used after, or combined with, behavioural therapy as first-line medication for OAB management.8 However, there is emerging evidence about the risks associated with long-term ACH use, particularly in older patients.15
| Cognitive impairment | Dementia |
The central nervous systems of older patients (>65 years) may be sensitive to ACH-related adverse effects.15,16 Use of medications with medium or high ACH activity was associated with deficits of memory, executive function, cognitive performance and increased risk of clinical cognitive impairment.17,18 | Higher cumulative ACH use in older patients was associated with an increased risk of dementia.8 Concurrent use of multiple ACH medications in older adults was associated with greater risk of hospitalisation for confusion.19 |
ACH, anticholinergic; OAB: overactive bladder, PTNS: percutaneous tibial nerve stimulation; SNM: sacral nerve modulation; UI: urinary incontinence.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250040.