This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
BOTOX®: For treating OAB5
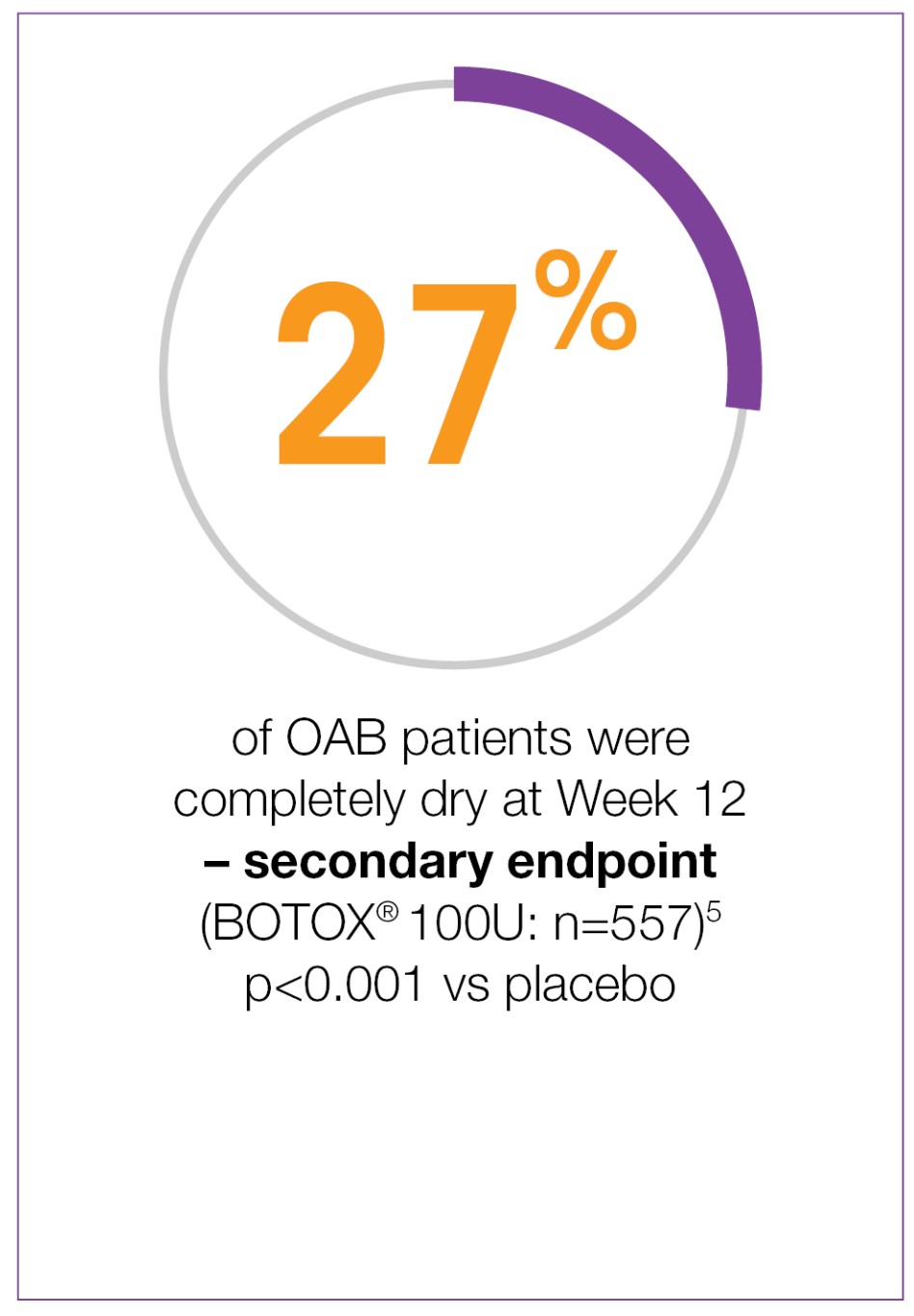
BOTOX® demonstrates significant and sustained improvements for OAB patients5-7
Week 12 co-primary endpoint results:
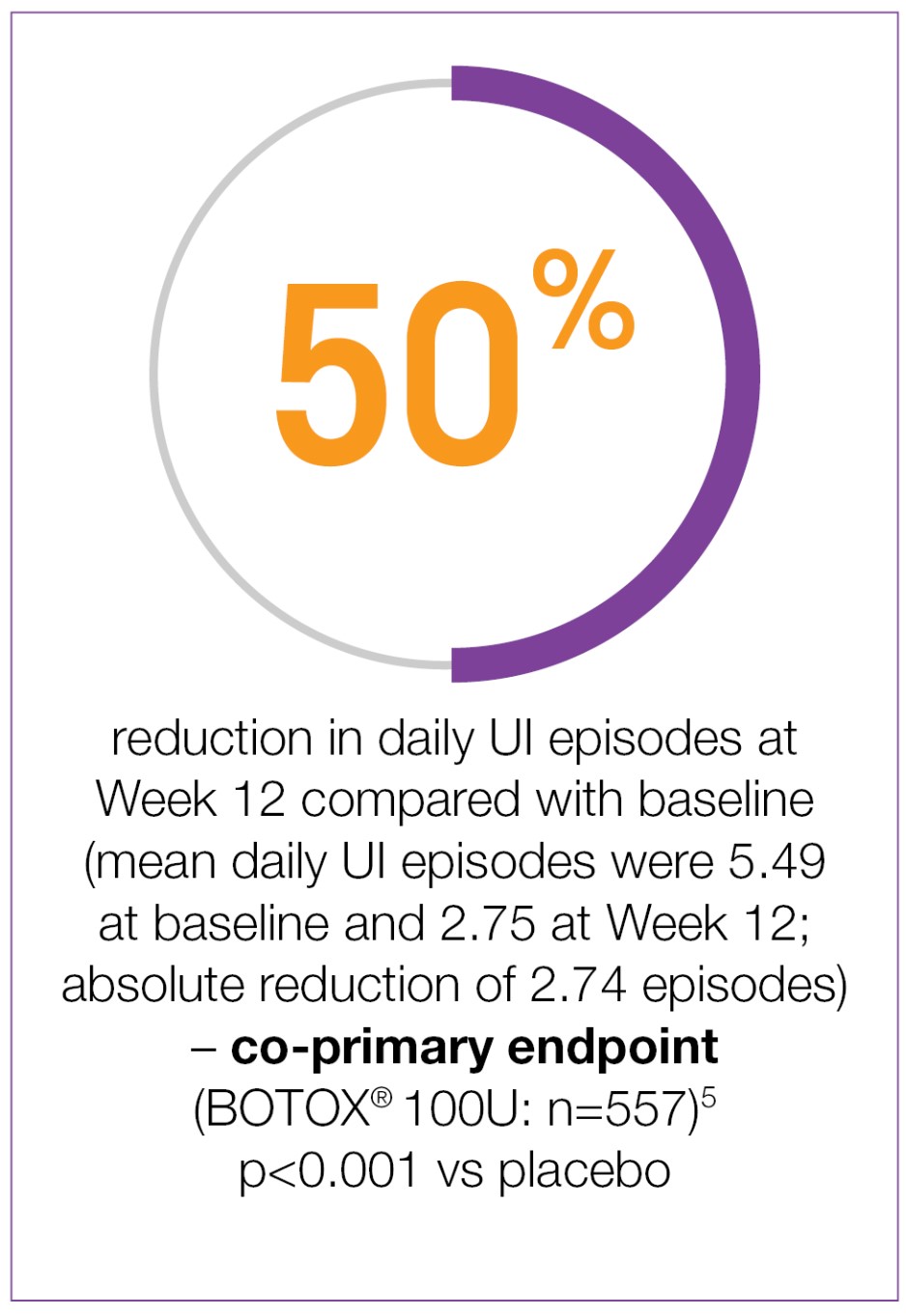
BOTOX® demonstrated significant reductions from baseline in daily UI episodes compared with placebo (-2.74 vs -0.95; p<0.001 vs placebo).5
Significantly greater proportion of BOTOX® patients achieved a positive response on the TBS compared to placebo (61.8% vs 28.0%; p<0.001 vs placebo).7
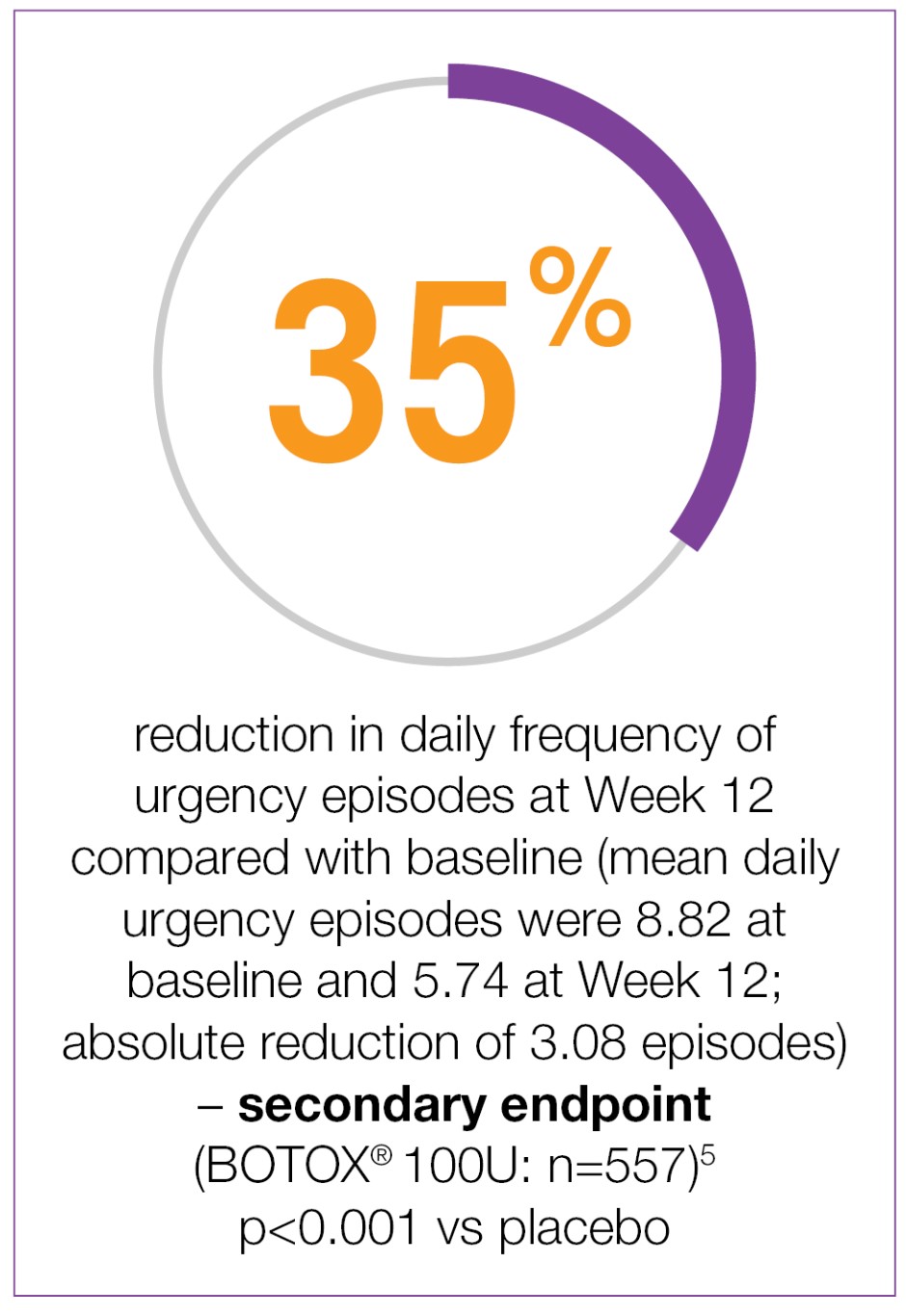
Study context: Two double-blind, placebo-controlled, randomised, 24-week phase 3 clinical studies were conducted in 1105 patients with overactive bladder, with symptoms of urge urinary incontinence, urgency and frequency, whose symptoms had not been adequately managed with at least one anticholinergic therapy. To evaluate the impact of efficacy, safety and health-related quality of life of BOTOX® in patients with OAB with UI. Patients were randomised to receive either 100U of BOTOX® (n=557) or placebo (n=548). Mean baseline for daily UI episodes: BOTOX® 100U 5.49/day, placebo 5.39/day. Mean baseline for daily urgency episodes: BOTOX® 100U 8.82/day, placebo 8.31/day.5
3 reasons to trust BOTOX® in your OAB patients
Over 3.5 years of continued BOTOX® treatment, durable improvements were seen in OAB symptoms and quality of life6
BOTOX® provides sustained reduction in daily episodes of UI and urgency for up to 6 cycles of treatment from baseline*6
BOTOX® is generally well tolerated in OAB with CIC rates that are low and generally transient5,7
Safety and tolerability experience from +30 years of use in a range of conditions2-4,8,9
*By comparison to placebo (p<0.001).5
CIC: clean intermittent catheterisation; OAB: overactive bladder; TBS: treatment benefit scale; UI: urinary incontinence.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250034.











.png)
.png)
.png)
.png)
.png)
.png)
