This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
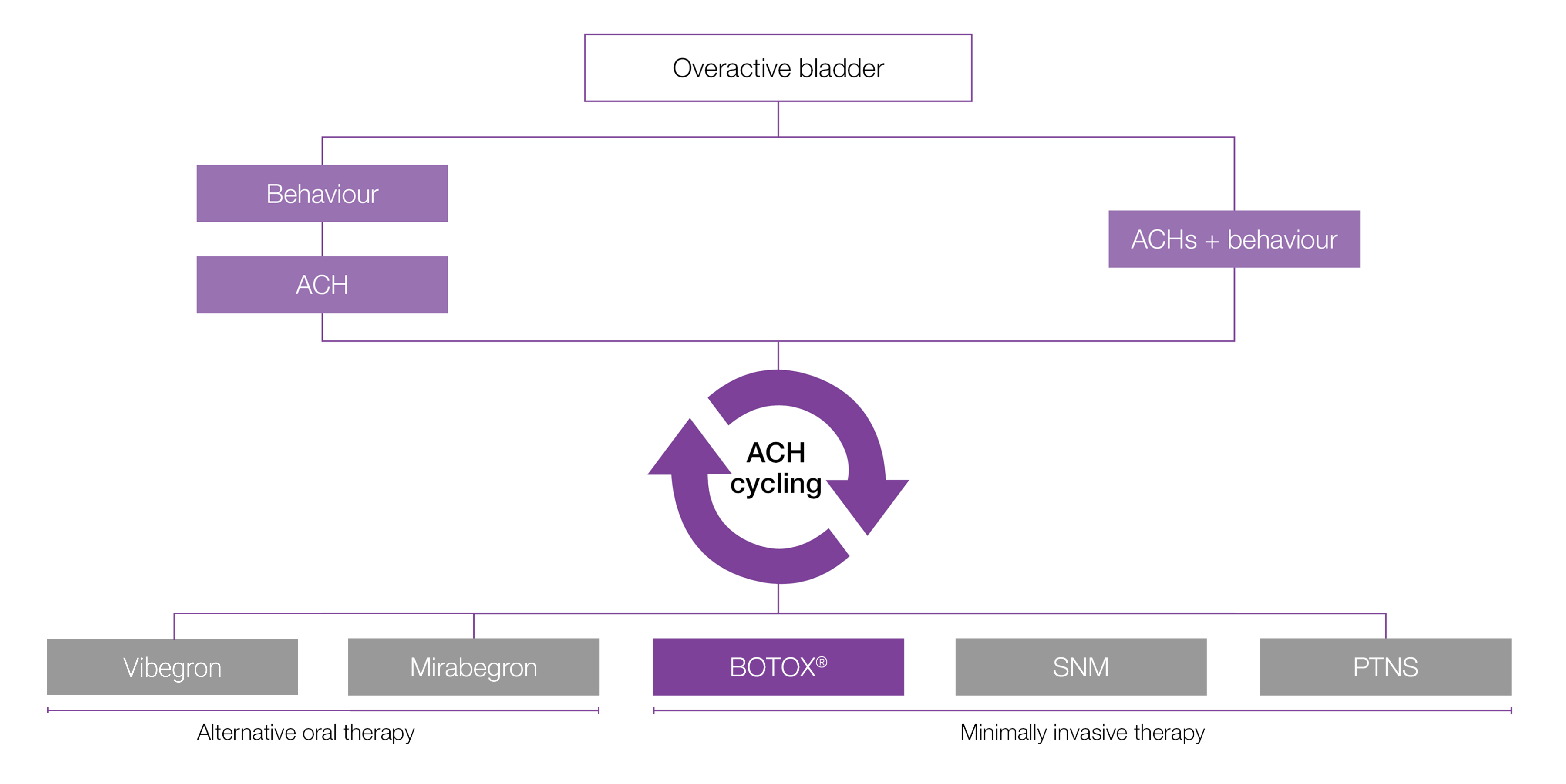
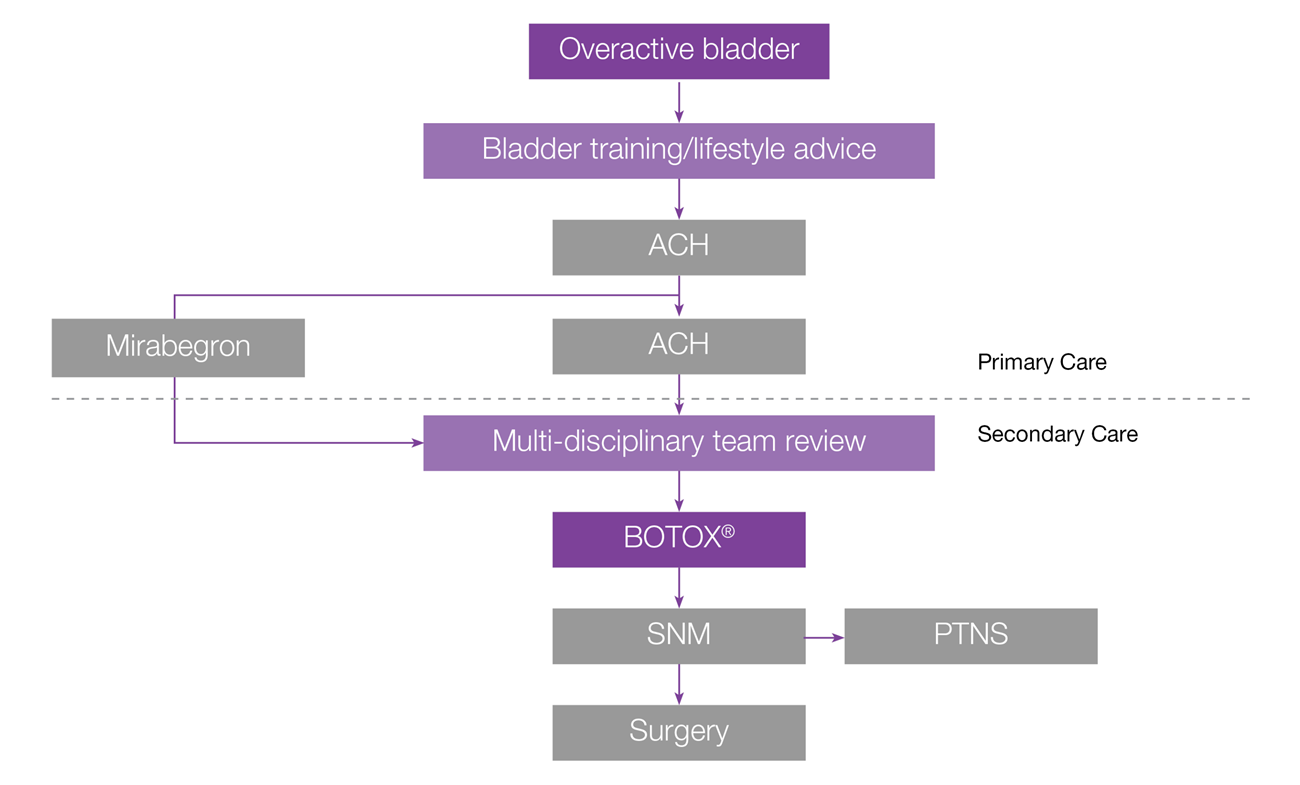
Considerations and guidance for using BOTOX® as a treatment option for managing overactive bladder (OAB)
ACH: anticholinergic; MDT, multi disciplinary team; NICE: National Institute for Health and Care Excellence; OAB: overactive bladder; SMC: Scottish Medicines Consortium
BOTOX® is accepted for restricted use for OAB within NHS Scotland:8
- BOTOX is accepted for restricted use within NHS Scotland for the management of bladder dysfunctions in adult patients who are not adequately managed with anticholinergics: overactive bladder with symptoms of urinary incontinence, urgency and frequency:
- SMC restriction: in patients who have failed appropriate oral treatment options.
NICE guidelines refer to the management of urinary incontinence in women only.7
NICE guidance:7
- 1.4.44 After a local MDT review, offer bladder wall injection with botulinum toxin type A to women with overactive bladder caused by detrusor overactivity that has not responded to non-surgical management, including pharmacological treatments.
- 1.4.45 Consider treatment with botulinum toxin type A after a local MDT review for women with symptoms of overactive bladder in whom urodynamic investigation has not demonstrated detrusor overactivity, if the symptoms have not responded to non-surgical management and the woman does not wish to have other invasive treatments.
- 1.4.46 After a local MDT review, discuss the benefits and risks of treatment with botulinum toxin type A with the woman and explain:
• the likelihood of complete or partial symptom relief
• the process of clean intermittent catheterisation, the risks, and how long it might need to be continued
• the risk of adverse effects, including an increased risk of urinary tract infection
• that there is not much evidence about how long the injections work for, how well they work in the long term and their long-term risks
©NICE [2019] Urinary incontinence and pelvic organ prolapse in women: management; NICE [2013] Mirabegron for treating symptoms of overactive bladder. Available from nice.org.uk. All rights reserved. Subject to Notice of rights.
NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BUO-250037.