This promotional material is intended for UK Healthcare Professionals only.
BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
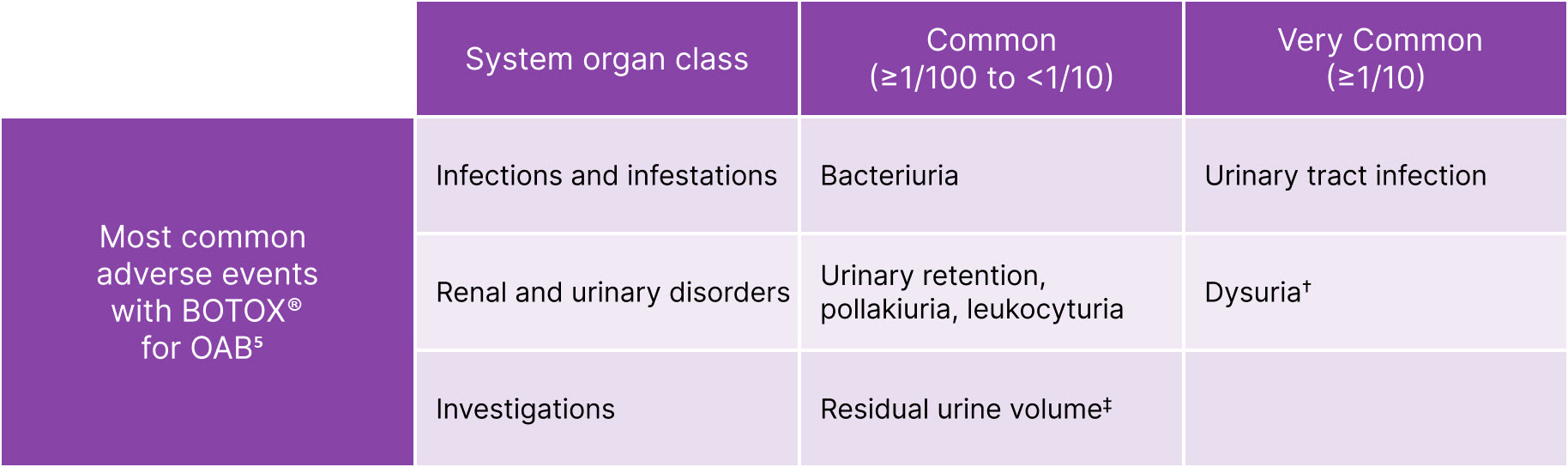
BOTOX® has safety and tolerability experience from +30 years of use in a range of conditions5-7
BOTOX® is generally well-tolerated in overactive bladder (OAB)5
Adapted from BOTOX® Summary of Product Characteristics.5
‡Elevated PVR not requiring catheterisation. †Procedure-related adverse reactions.
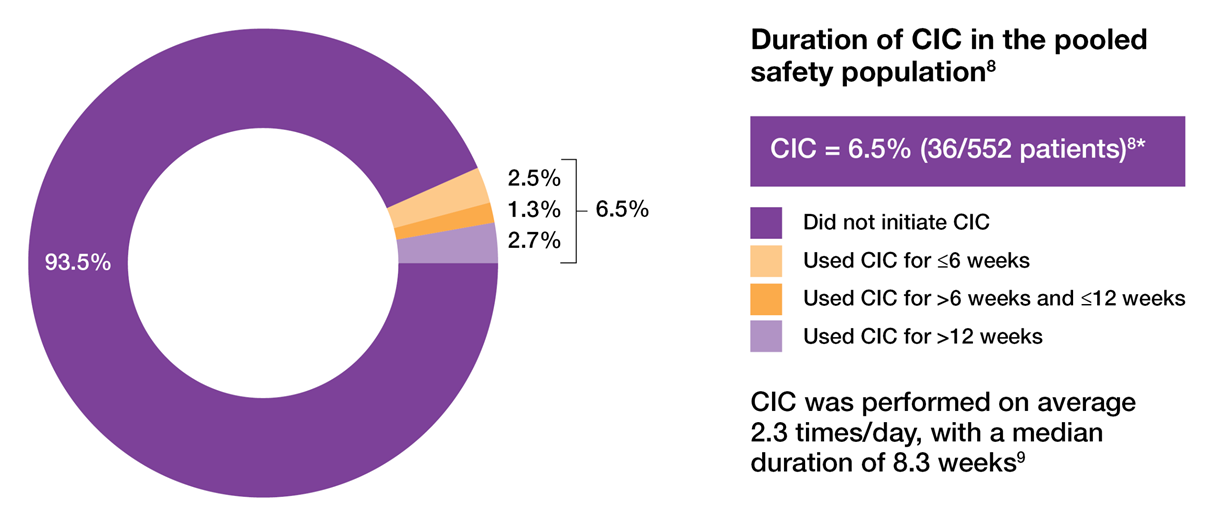
Clean intermittent catheterisation (CIC) rates that are low and predominantly transient8,9
CIC requirements and duration in two 24-week phase 3 trials of OAB patients treated with 100U BOTOX®8
Adapted from Sievert KD, et al. 2014.8
Study context: A prespecified pooled analysis of two placebo-controlled, phase 3 trials evaluated whether the number of prior anticholinergics used or reason for their discontinuation affected the treatment response to BOTOX® 100U in OAB patients with urinary incontinence. The co-primary endpoints were change from baseline at week 12 in urinary incontinence episodes/day and proportion of patients reporting a positive response on the treatment benefit scale. The most common AEs were localised urologic events. UTI was the most frequently reported AE in the pooled safety population and all but one case of UTI was uncomplicated.8
*Patients requiring CIC at any point during the treatment cycle. CIC was initiated if the PVR urine volume was ≥ 200ml and < 350ml with associated symptoms or if the PVR urine volume was ≥ 350ml, regardless of symptoms.8
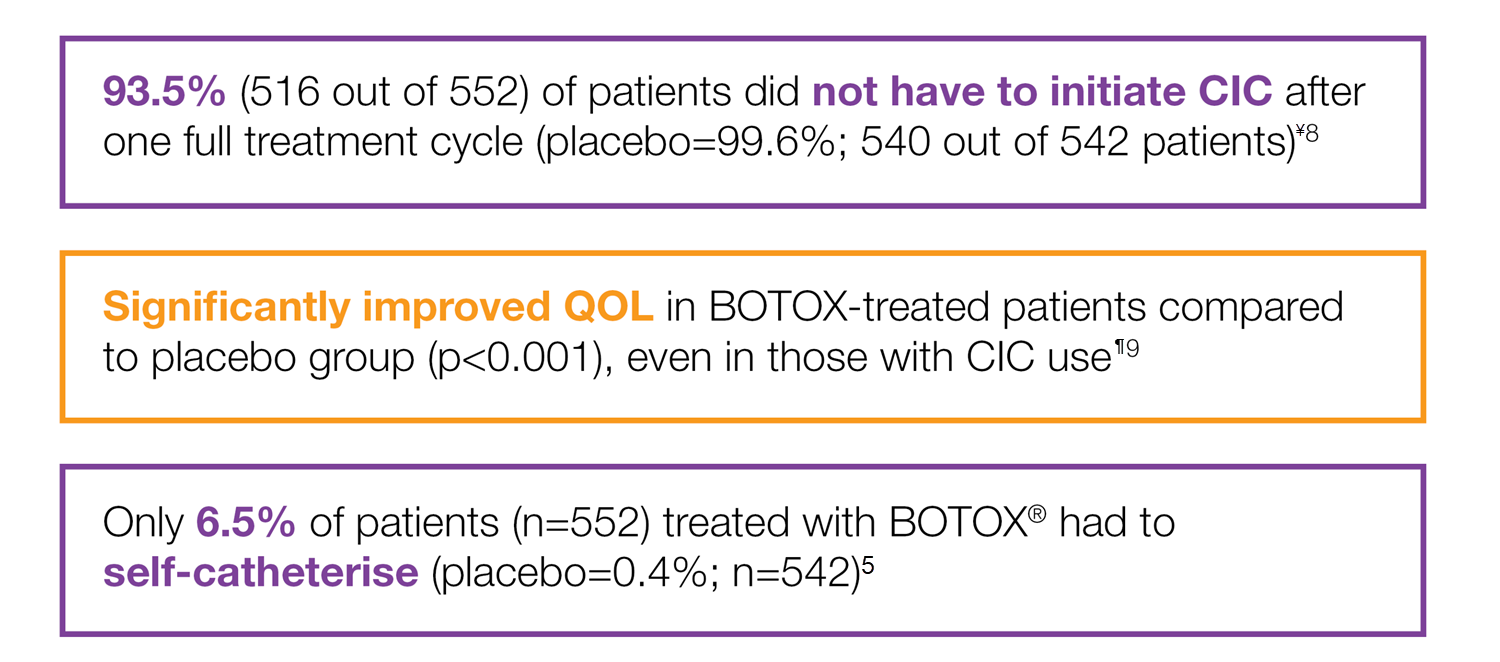
BOTOX® is generally well-tolerated in OAB5,8
¥Study context: A prespecified pooled analysis of two placebo-controlled, phase 3 trials evaluated whether the number of prior anticholinergics used or reason for their discontinuation affected the treatment response to BOTOX® 100U in OAB patients with urinary incontinence.8
¶Study context: A pooled analysis of two randomised controlled trials, to evaluate the impact of BOTOX® on quality of life and practical aspects of daily living. The co-primary endpoints were the mean reduction in UI episodes per day and the proportion of patients reporting a positive response on the treatment benefit scale.9
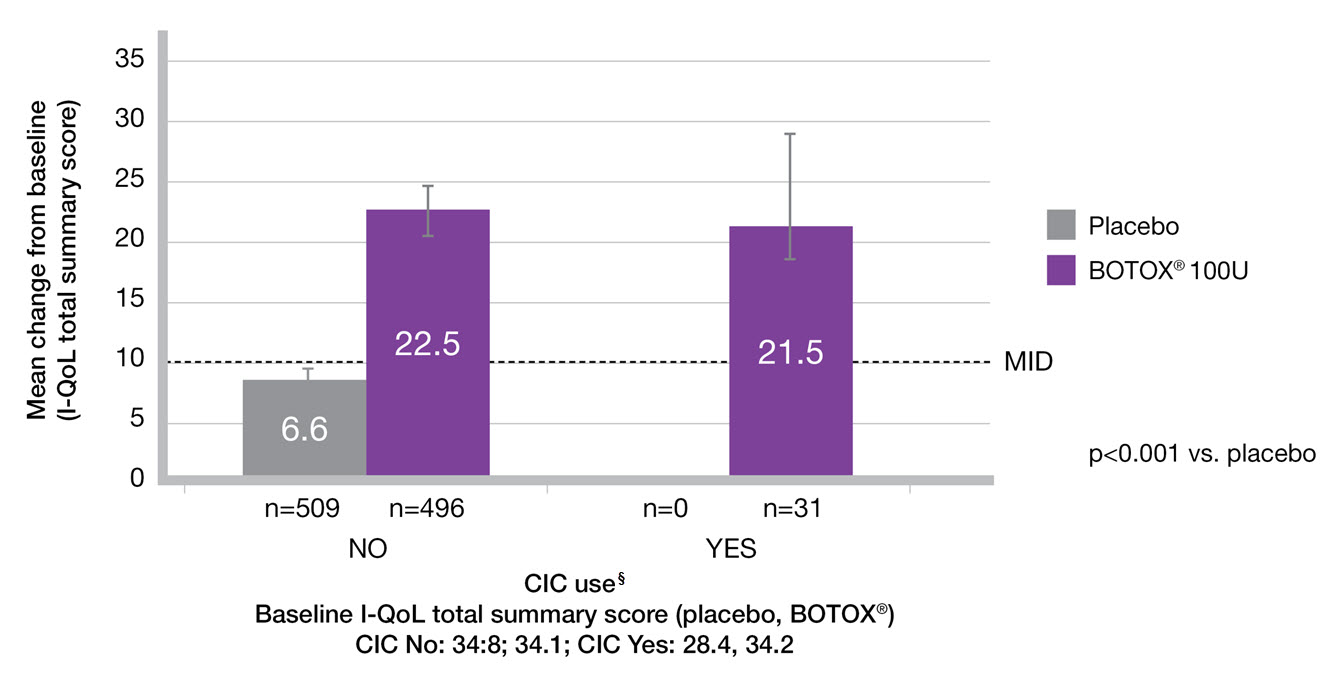
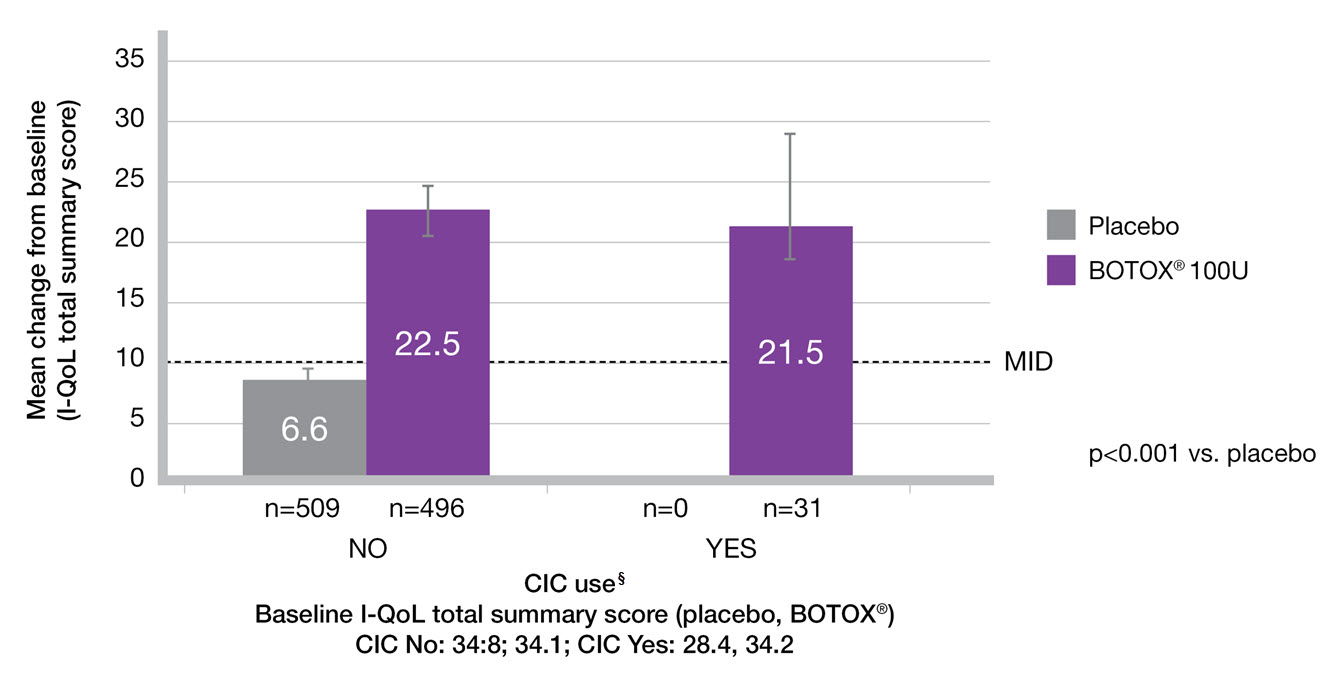
CIC does not impact upon QoL improvement9
Adapted from Everaert K, et al. 2015.9
Study context: A pooled analysis of two randomised controlled trials, to evaluate the impact of BOTOX® on quality of life and practical aspects of daily living. Change from baseline in I-QoL total summary score in the subgroups by CIC use.9
§The n values denote the number of patients with data available at Week 12. Error bars represent 95% confidence intervals.
CIC: clean intermittent catheterisation; I-QoL: incontinence quality of life; MID, minimal important difference; OAB: overactive bladder; PVR: post-void residual volume; QoL: quality of life; UTI: urinary tract infection.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: May 2025. UK-BUO-250036.