Note to affiliate: Please update the brand name and generic name as part of your local approval designations.
Please refer to the PRODUODOPA Summary of Product Characteristics (SmPC) for complete Prescribing and Safety Information.
Note to affiliate: The information within this website is based on the draft SmPC submitted to MPA on 3 March 2023. This website also includes 52-week 741 study data from the third interim analysis that was published 25 August 2023, and 12-week analysis from the 736 study. Local review and approval will be needed prior launching this website. Please refer to the Campaign Guidance Document developed for further guidance on key claims utilized within this piece. This piece also includes pump information, which is intended for use post drug and device approval or as appropriate based on country-specific regulations.
First and Only
A new possible course of action as the first-and-only continuous subcutaneous levodopa-based therapy for patients with advanced Parkinson’s disease.1-3
PRODUODOPA is indicated for the treatment of advanced levodopa-responsive PD with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson’s medicinal products have not given satisfactory results.1
Continuous and Personalized
PRODUODOPA provides a wide range of customizable dosing, delivered via a single infusion site and canula that can remain in place for up to 3 days, to accommodate individual patient needs.1§
PRODUODOPA can be taken alone or, if necessary, with other concurrent medicinal products for PD, based on the judgment of the HCP.1
*More refers to improvement from baseline compared with oral IR levodopa/carbidopa in “On” and “Off” time at Week 12.1 Tap the underlined link to see data.
†Patients made an entry in a PD diary upon waking and every 30 minutes during their normal waking time, and upon awakening from time asleep.1,2
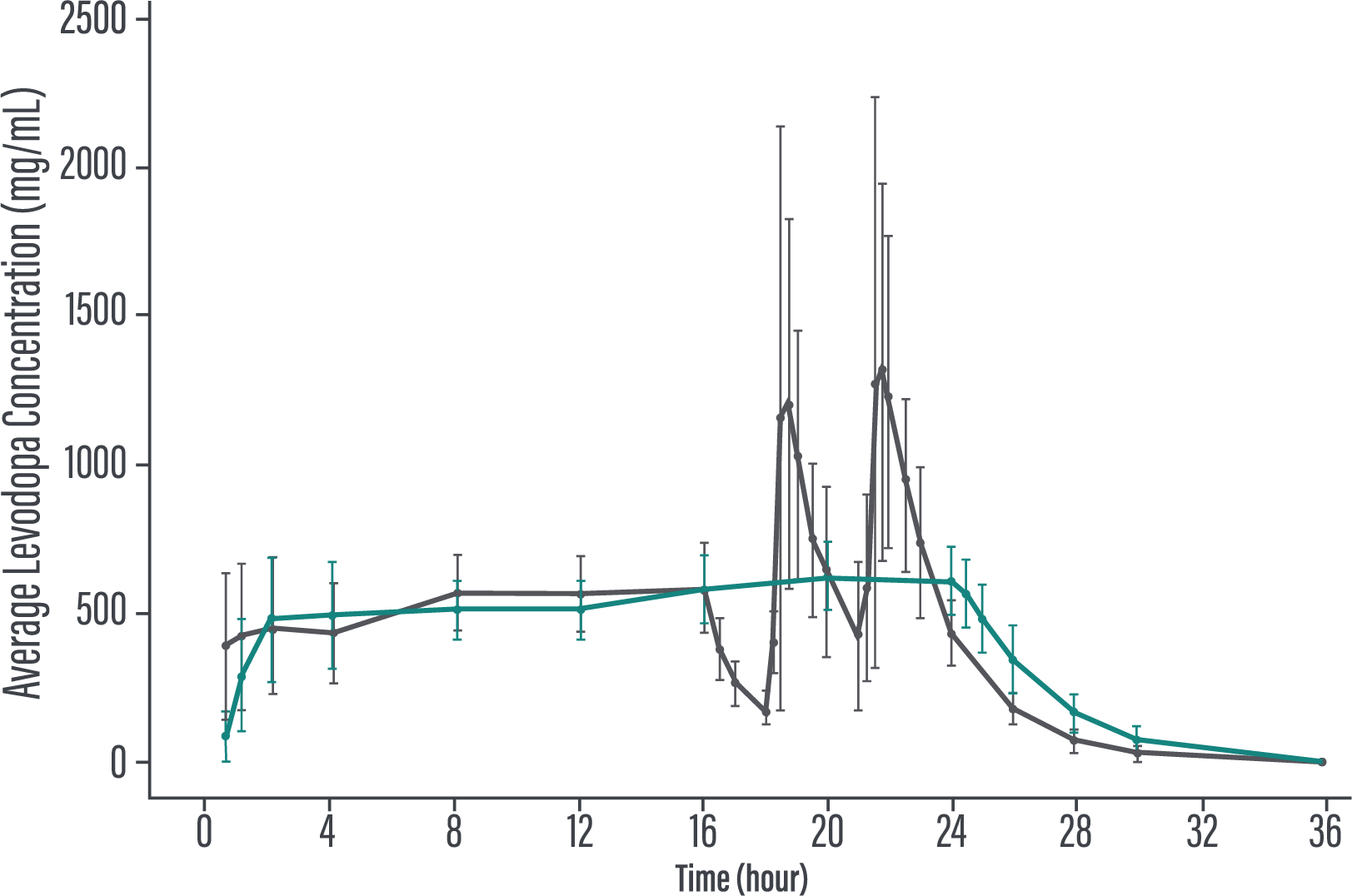
‡PRODUODOPA is a levodopa-based therapy delivered subcutaneously as a 24-hour infusion.1,2
§Rotate the infusion set and use a new infusion set at least every 3 days.1 PRODUODOPA allows for dosing up to 4260 mg levodopa/day with 3 programmable flow rates (base, high, and low) and an extra-dose capability. Infusion rates may be adjusted in increments as small as 0.01 mL/hour (~1.7 mg of levodopa/hour).1
HCP=healthcare professional; IR=immediate release; PD=Parkinson’s disease.
*Consider PRODUODOPA for patients with advanced levodopa-responsive PD with severe motor fluctuations and hyperkinesia when available PD medicine combinations have not given satisfactory results, who want more "On" time in their day†
†MORE refers to "On" time compared to oral immediate-release (IR) levodopa/carbidopa.1
Note to affiliate: The intent of the 3 patient profiles is that each customer sees all 3 patient profiles, either in one call or during the course of several calls. When utilizing the patient profiles, it is important to discuss the profiles for James and Hina first, before reviewing the profile for Jean.
PRODUODOPA is indicated for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.
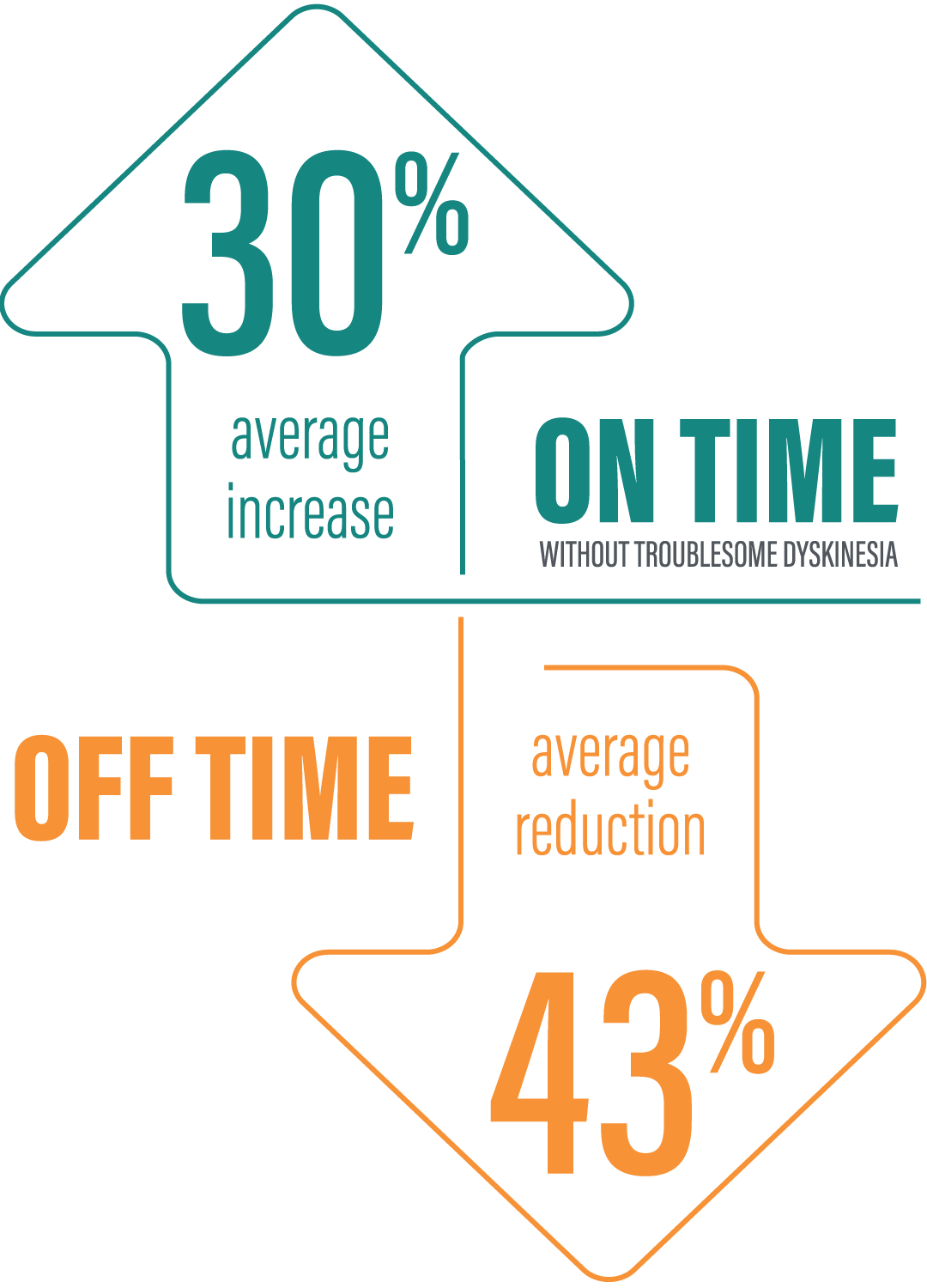
*“On” time without troublesome dyskinesia is the sum of “On” time without dyskinesia and “On” time with non‑troublesome dyskinesia.2
†Based on the PD diary, the average daily normalized “On” time without troublesome dyskinesia for patients on PRODUODOPA was 9.20 (+/-2.42) hours at baseline and increased by 2.72 (+/-0.52) hours at Week 12 compared with an increase of 0.97 (+/-0.50) hours at Week 12 from a baseline of 9.49 (+/-2.62) hours for patients taking optimized IR levodopa/carbidopa (LS mean change [SE]). This resulted in a statistically significant improvement of 1.75 (+/-0.65) hours in patients on PRODUODOPA vs oral IR LD/CD (LS mean of difference [SE]; P=0.0083). Based on the PD diary, the average daily normalized “Off” time for patients on PRODUODOPA was 6.34 (+/-2.27) hours at baseline and decreased by 2.75 (+/-0.50) hours at Week 12 compared with a decrease of 0.96 (+/-0.49) hours at Week 12 from a baseline of 5.91 (+/-1.88) hours for patients taking optimized IR levodopa/carbidopa (LS mean change [SE]). This resulted in a statistically significant improvement of 1.79 (+/-0.63) hours in patients on PRODUODOPA vs oral IR LD/CD (LS mean of difference [SE]; P=0.0054).1
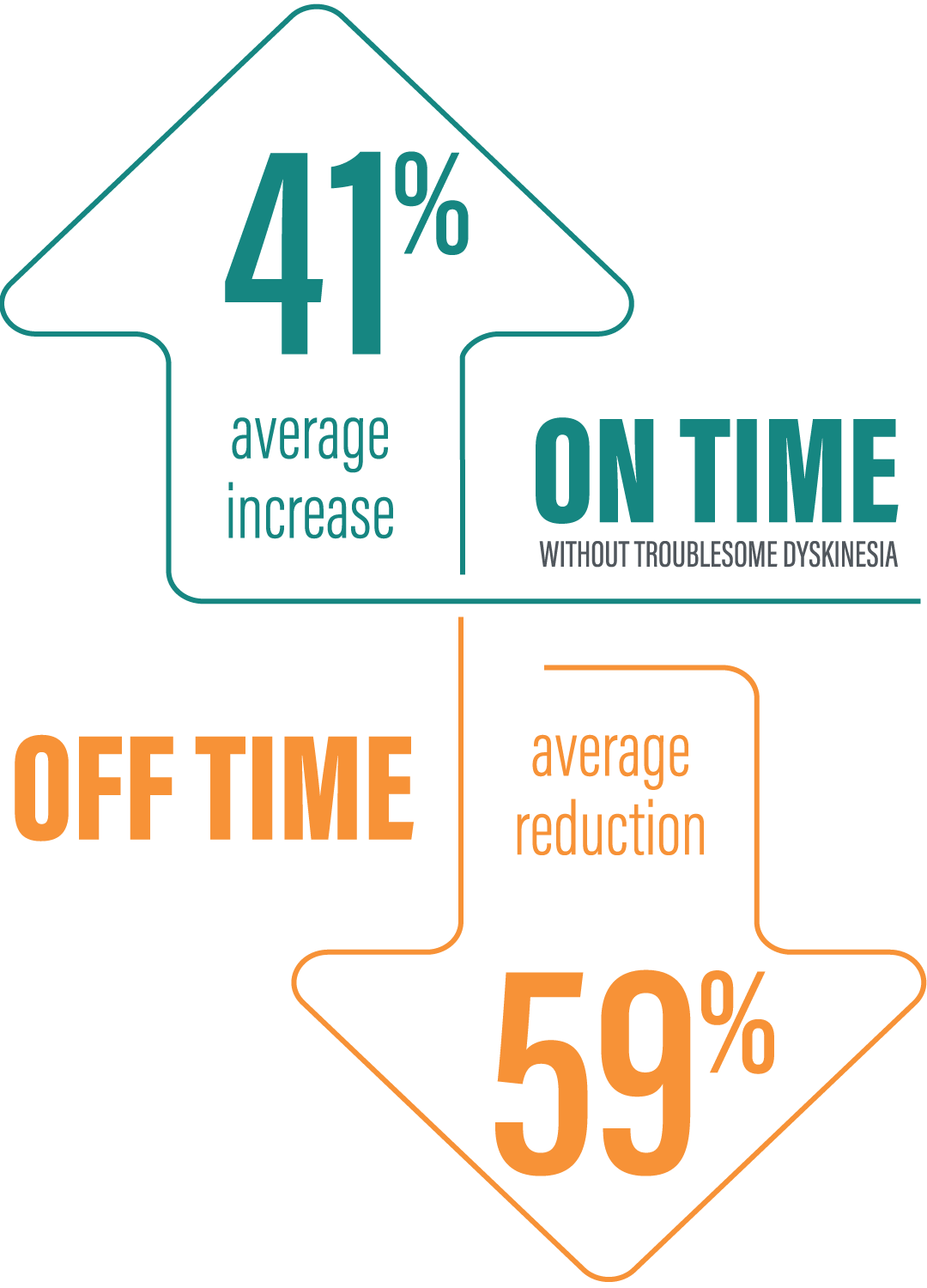
‡“On” time without troublesome dyskinesia improved by an average of 3.8 hours by Week 52 (mean 12.9 hours) compared to baseline (mean 9.1 hours) based on the PD diary (N=104). “Off” time was decreased by an average of 3.5 hours by Week 52 (mean 2.5 hours) compared to baseline (mean 6.0 hours) based on the PD diary (N=104).1,6
§Data presented reflect the third interim analysis of 52-week study results that includes 104 patients. This study is ongoing.1
IR=immediate release; LS=least squares; SE=standard error; PD=Parkinson's disease.
*For interruptions longer than 1 hour, or same infusion site/cannula used continuously for up to 3 days, a new infusion set (tubing and cannula) should be used and rotated to a different infusion site.
Learn more about your options for starting and optimizing treatment
Are You Ready?
PRODUODOPA is indicated for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson’s medicinal products have not given satisfactory results.1
Please refer to the PRODUODOPA SmPC for complete Prescribing and Safety Information.
REFERENCES:
- [DRAFT] Produodopa® (foslevodopa/foscarbidopa solution for infusion) Summary of Product Characteristics (SmPC). <insert current SmPC date>.
- Soileau MJ, Aldred J, Budur K, et al. Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022;21(12):1099-1109. doi:10.1016/S1474-4422(22)00400-8. Erratum in: Lancet Neurol. 2023;22(3):e5.
- Rosebraugh M, Liu W, Neenan M, Facheris MF. Foslevodopa/foscarbidopa is well tolerated and maintains stable levodopa and carbidopa exposure following subcutaneous infusion. J Parkinsons Dis. 2021;11(4):1695-1702. doi:10.3233/JPD-212813
- Lundqvist C. Continuous levodopa for advanced Parkinson’s disease. Neuropsychiatr Dis Treat. 2007;3(3):335-348.
- Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239-1252. doi:10.2174/1570159X15666170510143821
- Aldred J, Freire-Alvarez E, Amelin AV, et al. Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson's Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study. Neurol Ther. 2023. doi: 10.1007/s40120-023-00533-1. Epub ahead of print.
- Rosebraugh M, Stodtmann S, Liu W, Facheris MF. Foslevodopa/foscarbidopa subcutaneous infusion maintains equivalent levodopa exposure to levodopa-carbidopa intestinal gel delivered to the jejunum. Parkinsonism Relat Disord. 2022;97:68-72.
- [DRAFT]Produodopa® (foslevodopa/foscarbidopa solution for infusion) Patient Pump Instructions for Use. <insert current patient IFU date>.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
PRODUODOPA® Indication and Summary of Important Treatment Considerations1
Indication:
Treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.
Contraindications:
PRODUODOPA is contraindicated in patients with hypersensitivity to the active substances or to any of the excipients, narrow-angle glaucoma, severe heart failure, acute stroke, severe cardiac arrhythmia, co-medication with selective type A inhibitors and nonselective MAO inhibitors, conditions contraindicated for adrenergics (e.g. pheochromocytoma, hyperthyroidism, and Cushing’s syndrome), and suspicious skin lesions or history of melanoma.
Please refer to the Produodopa SmPC for complete Prescribing and Safety Information.
<placeholder link for local PRODUODOPA SmPC>
ALL-PRODD-220020. Date of preparation: July 2023.