Continuous and Personalized
PRODUODOPA provides a wide range of customizable dosing, delivered via a single infusion site and canula that can remain in place for up to 3 days, to accommodate individual patient needs.1§
PRODUODOPA can be taken alone or, if necessary, with other concurrent medicinal products for PD, based on the judgment of the HCP.1
*More refers to improvement from baseline compared with oral IR levodopa/carbidopa in “On” and “Off” time at Week 12.1 Tap the underlined link to see data.
†Patients made an entry in a PD diary upon waking and every 30 minutes during their normal waking time, and upon awakening from time asleep.1,3
‡PRODUODOPA is a levodopa-based therapy delivered subcutaneously as a 24-hour infusion.1,3
§Rotate the infusion set and use a new infusion set at least every 3 days.1 PRODUODOPA allows for dosing up to 4260 mg levodopa/day with 3 programmable flow rates (base, high, and low) and an extra-dose capability. Infusion rates may be adjusted in increments as small as 0.01 mL/hour (~1.7 mg of levodopa/hour).1
HCP=healthcare professional; IR=immediate release; PD=Parkinson's disease; PK=pharmacokinetics.
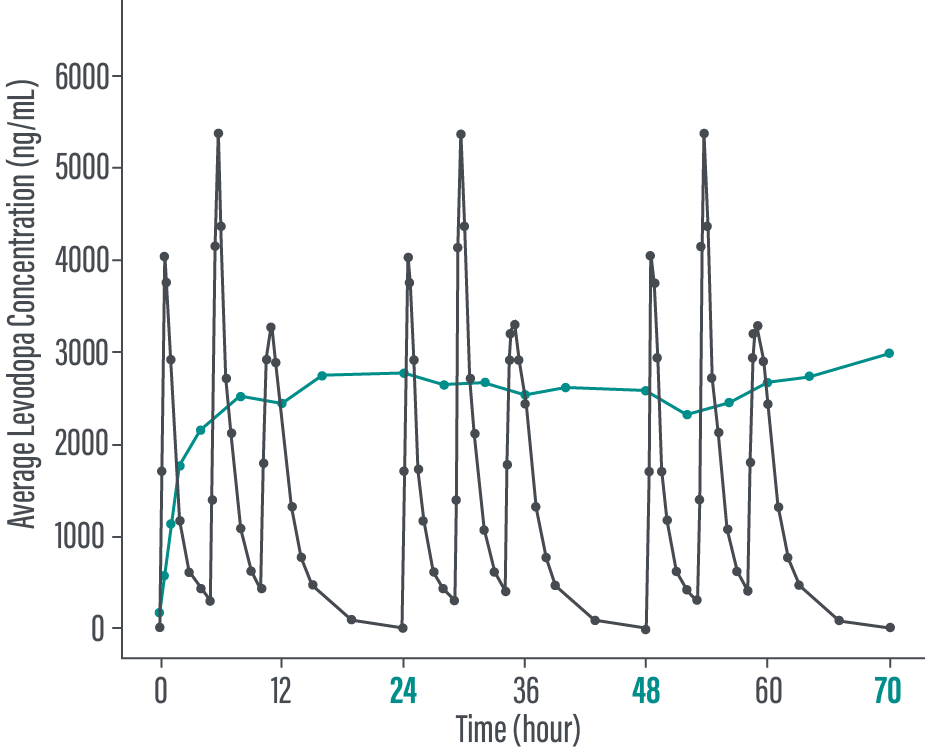
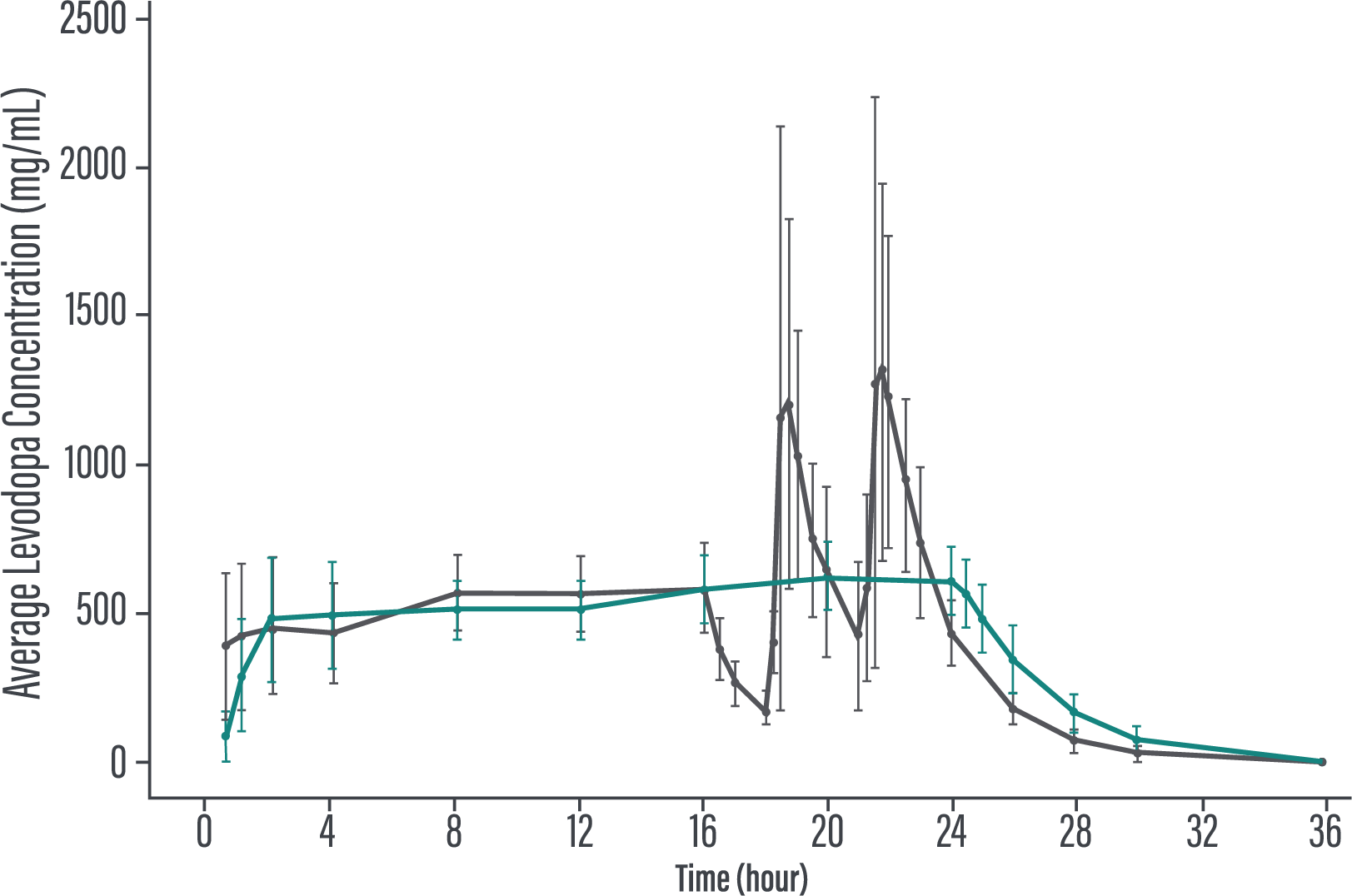
Levodopa exposure following PRODUODOPA infusion in PD patients compared to oral levodopa/carbidopa dosing
IITo assess PK fluctuation differences, the average levodopa and carbidopa exposures following PRODUODOPA infusion were compared to simulated exposure of daily doses of 400/100 mg levodopa/carbidopa TID.
IR=immediate release; LC/CD=levodopa/carbidopa; TID=three times a day.
Adapted from Rosebraugh, 2021.2
Note to affiliate: The graph presented here based on the published Rosebraugh 2021 study, however the colors have been adjusted to match the project. Please update as per your local rules and regulations.
*In the PK comparability study, PRODUODOPA was administered as a continuous subcutaneous infusion, 24 hours per day. In healthy volunteers, steady state was achieved within 2 hours when PRODUODOPA was delivered as a loading dose followed by continuous infusion. Steady state was maintained during the infusion period.1
Note to affiliate: Click through this button to review primary and secondary endpoints from DUODOPA data.
When treated with PRODUODOPA, patients experienced more “On” time and less “Off” time.1*†
Continue to Clinical Profile page
*In the PRODUODOPA pivotal study at Week 12, “On” time without troublesome dyskinesia for patients on PRODUODOPA increased 2.72 hours from baseline compared with an increase from baseline of 0.97 hours for patients taking oral IR levodopa/carbidopa.1
†In the PRODUODOPA safety study, at Week 52 compared to baseline, “On” time without troublesome dyskinesia improved by an average of 3.8 hours (N=104); “Off” time decreased by an average of 3.5 hours (N=104).1,5
Please refer to the PRODUODOPA SmPC for complete Prescribing and Safety Information.
REFERENCES:
- [DRAFT] Produodopa® (foslevodopa/foscarbidopa solution for infusion) Summary of Product Characteristics (SmPC). <insert current SmPC date>.
- Rosebraugh M, Liu W, Neenan M, Facheris MF. Foslevodopa/foscarbidopa is well tolerated and maintains stable levodopa and carbidopa exposure following subcutaneous infusion. J Parkinsons Dis. 2021;11(4):1695-1702. doi:10.3233/JPD-212813
- Soileau MJ, Aldred J, Budur K, et al. Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022;21(12):1099-1109. doi:10.1016/S1474-4422(22)00400-8. Erratum in: Lancet Neurol. 2023;22(3):e5.
- Rosebraugh M, Stodtmann S, Liu W, Facheris MF. Foslevodopa/foscarbidopa subcutaneous infusion maintains equivalent levodopa exposure to levodopa-carbidopa intestinal gel delivered to the jejunum. Parkinsonism Relat Disord. 2022;97:68-72.
- Aldred J, Freire-Alvarez E, Amelin AV, et al. Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson's Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study. Neurol Ther. 2023. doi:10.1007/s40120-023-00533-1. Epub ahead of print. PMID: 37632656.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
PRODUODOPA® Indication and Summary of Important Treatment Considerations1
Indication:
Treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.
Contraindications:
PRODUODOPA is contraindicated in patients with hypersensitivity to the active substances or to any of the excipients, narrow-angle glaucoma, severe heart failure, acute stroke, severe cardiac arrhythmia, co-medication with selective type A inhibitors and nonselective MAO inhibitors, conditions contraindicated for adrenergics (e.g. pheochromocytoma, hyperthyroidism, and Cushing’s syndrome), and suspicious skin lesions or history of melanoma.
Please refer to the Produodopa SmPC for complete Prescribing and Safety Information.
<placeholder link for local PRODUODOPA SmPC>
ALL-PRODD-220020. Date of preparation: July 2023.