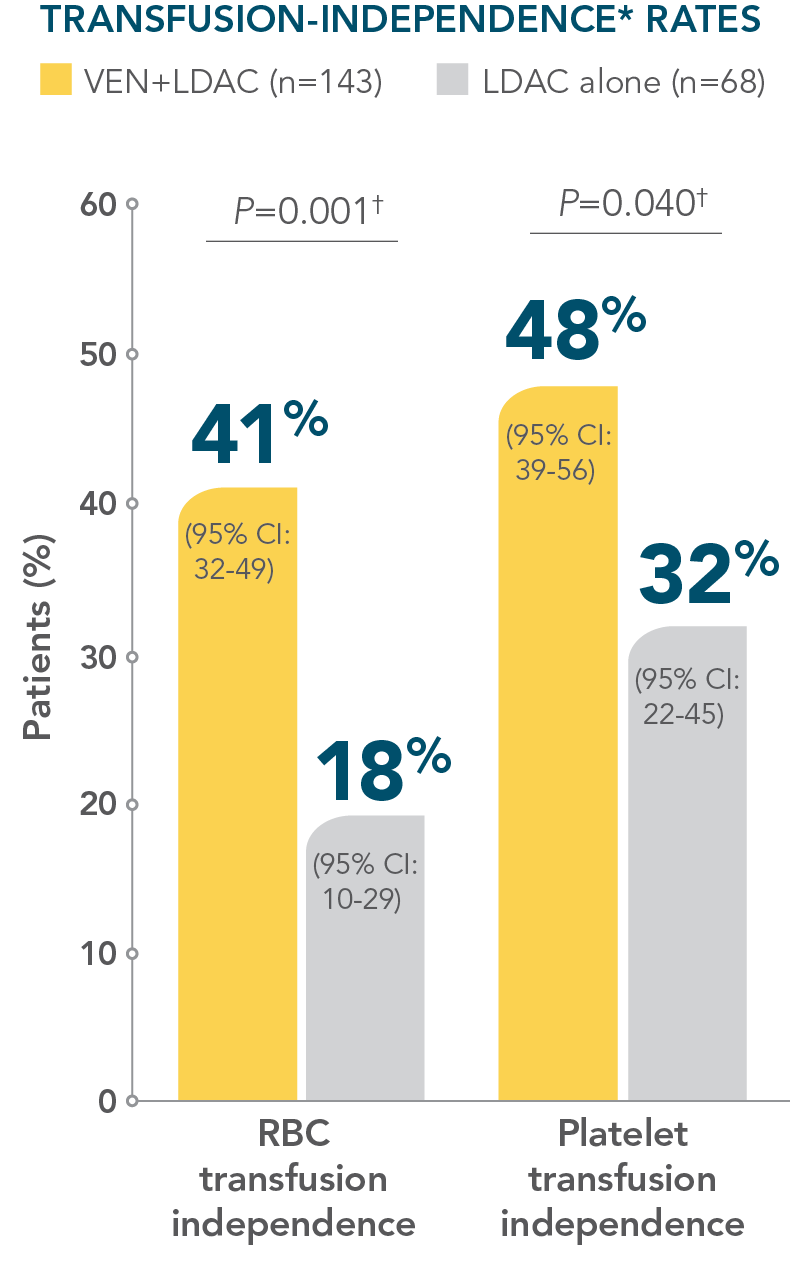For newly diagnosed patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy1
VENCLYXTO is the first approved BCL-2 inhibitor for the treatment of AML1
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
*VIALE-A was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus AZA in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus AZA was 14.7 months (95% CI: 11.9-18.7) vs 9.6 months for AZA alone (95% CI: 7.4-12.7) (HR=0.66 [95% CI: 0.52-0.85; P<0.001]).1
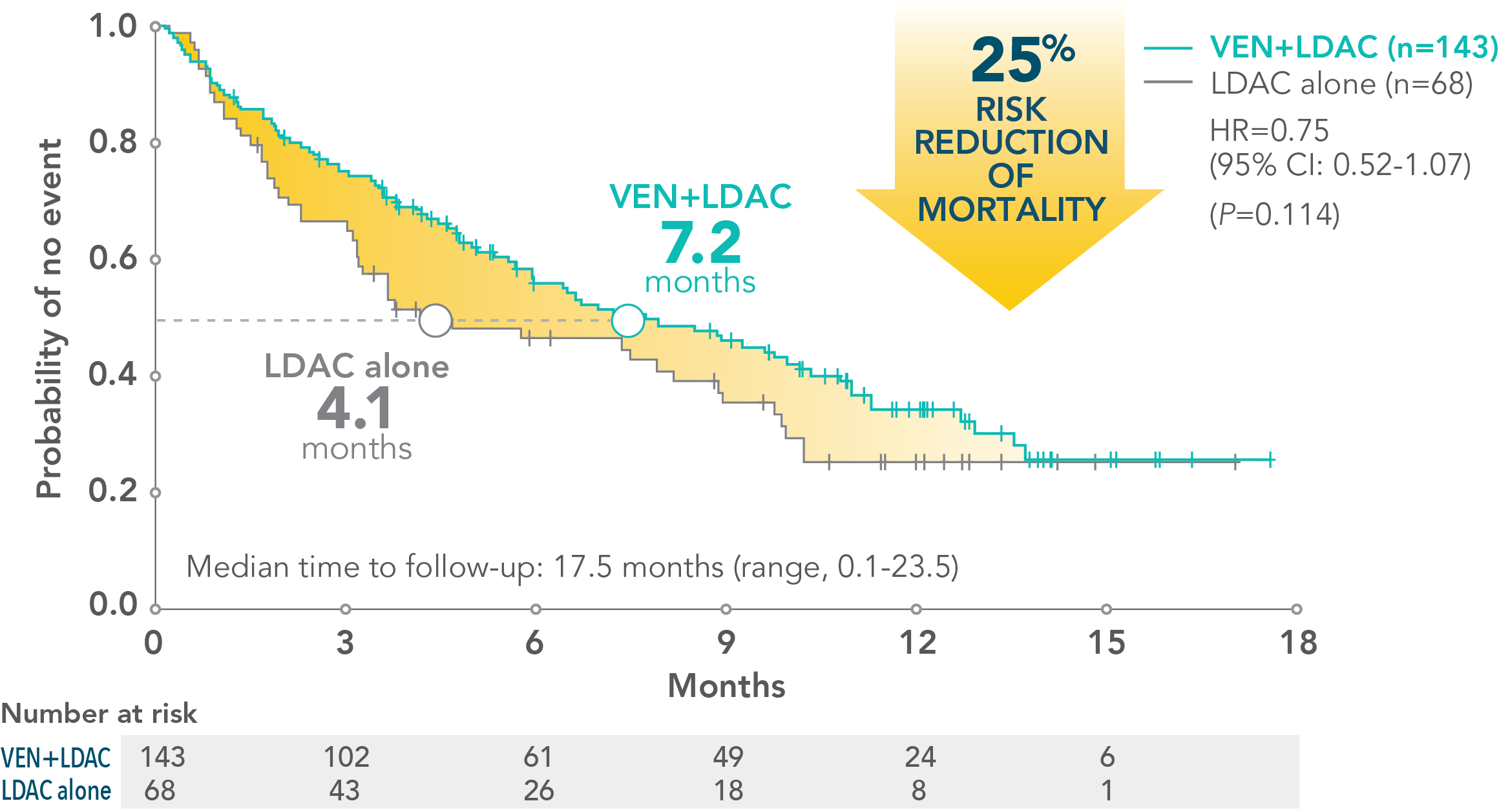
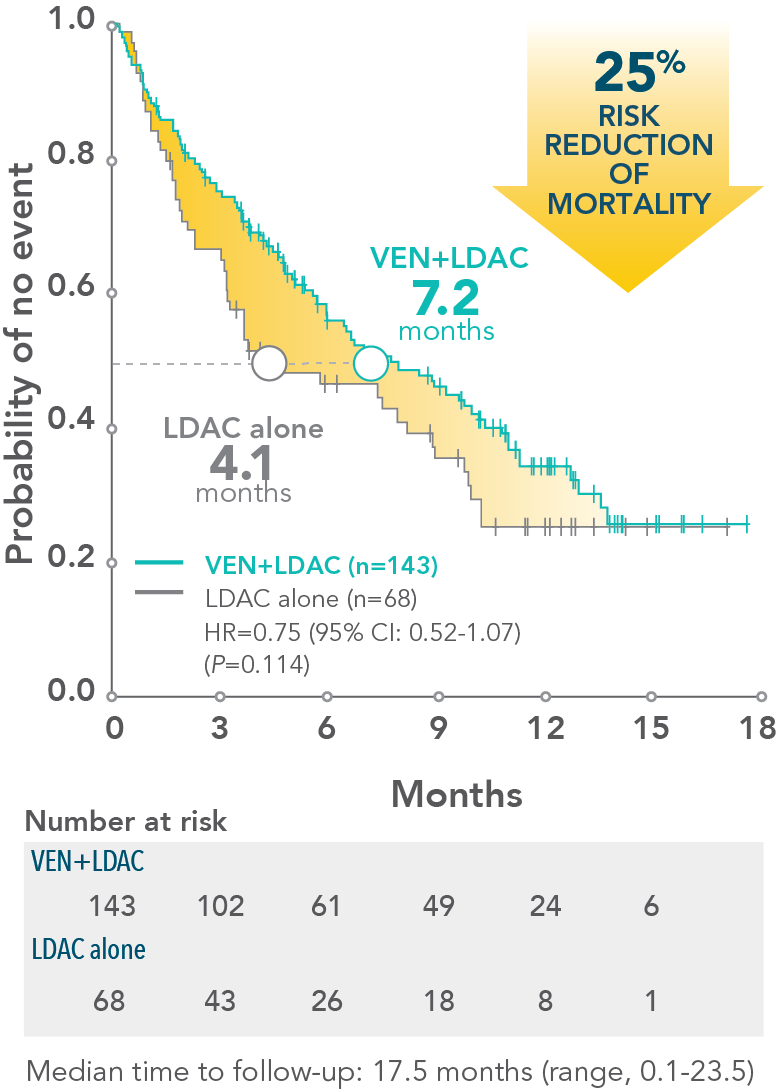
†VIALE-C was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus LDAC was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07; P=0.114]).1
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
BCL-2=B-cell lymphoma 2; AZA=azacitidine; LDAC=low-dose cytarabine; CI=confidence interval; HR=hazard ratio.
For newly diagnosed patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy1
VENCLYXTO is the first approved BCL-2 inhibitor for the treatment of AML1
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
*VIALE-A was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus AZA in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus AZA was 14.7 months (95% CI: 11.9-18.7) vs 9.6 months for AZA alone (95% CI: 7.4-12.7) (HR=0.66 [95% CI: 0.52-0.85; P<0.001]).1
†VIALE-C was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus LDAC was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07; P=0.114]).1
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
BCL-2=B-cell lymphoma 2; AZA=azacitidine; LDAC=low-dose cytarabine; CI=confidence interval; HR=hazard ratio.
*VIALE-C was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus LDAC was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07; P=0.114]).1
Increased median overall survival
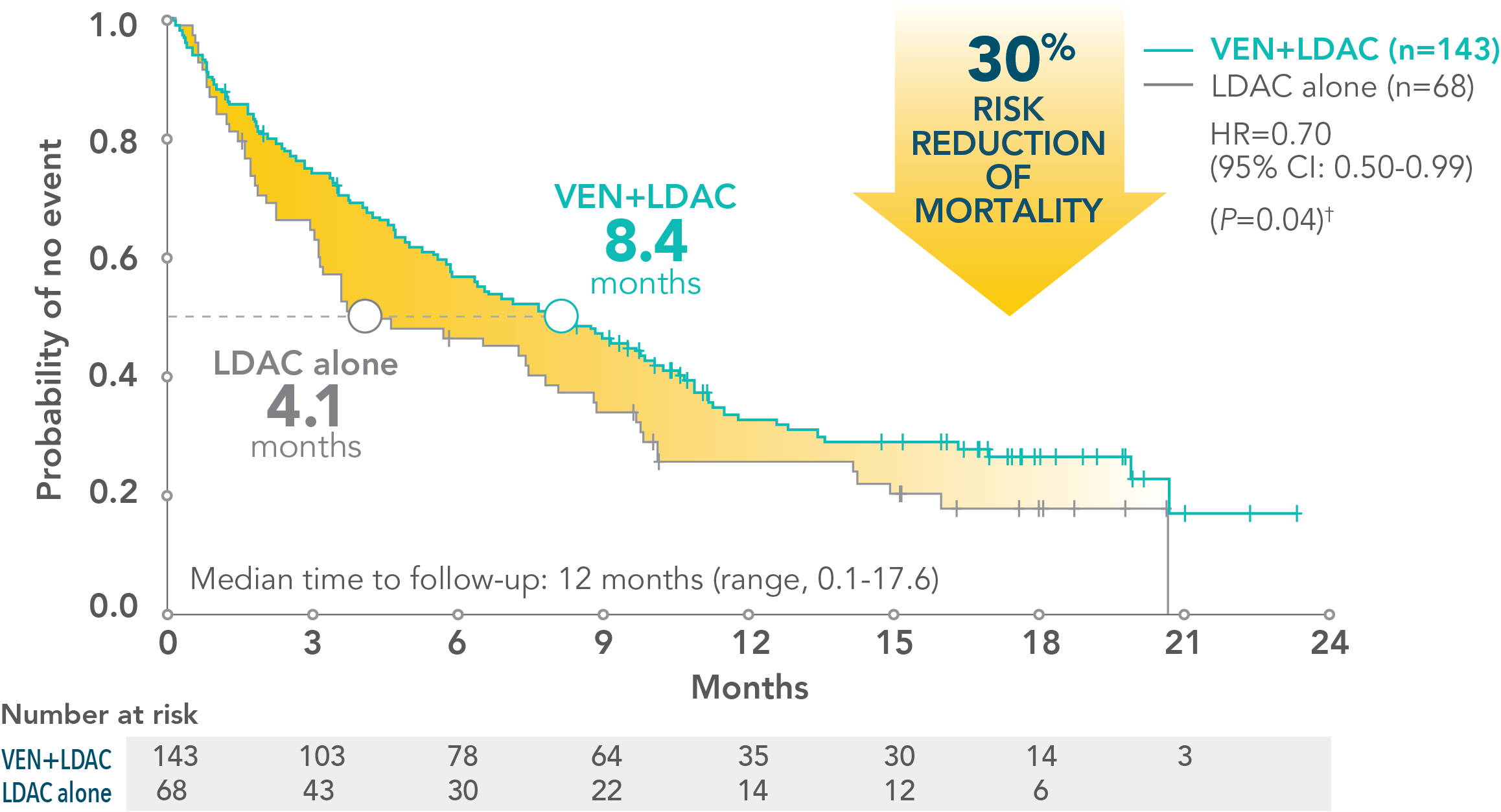
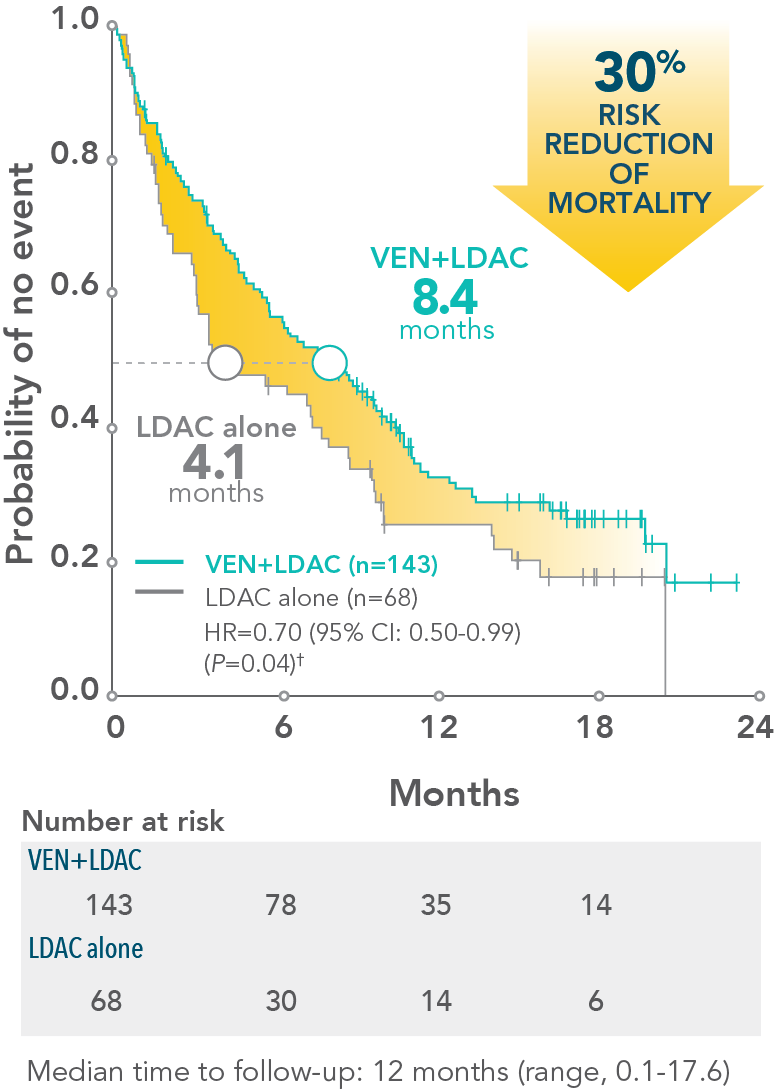
- 3.1-month increase in median overall survival with VENCLYXTO plus LDAC vs LDAC alone (7.2 months vs 4.1 months, respectively; HR=0.75 [95% CI: 0.52-1.07; P=0.114])
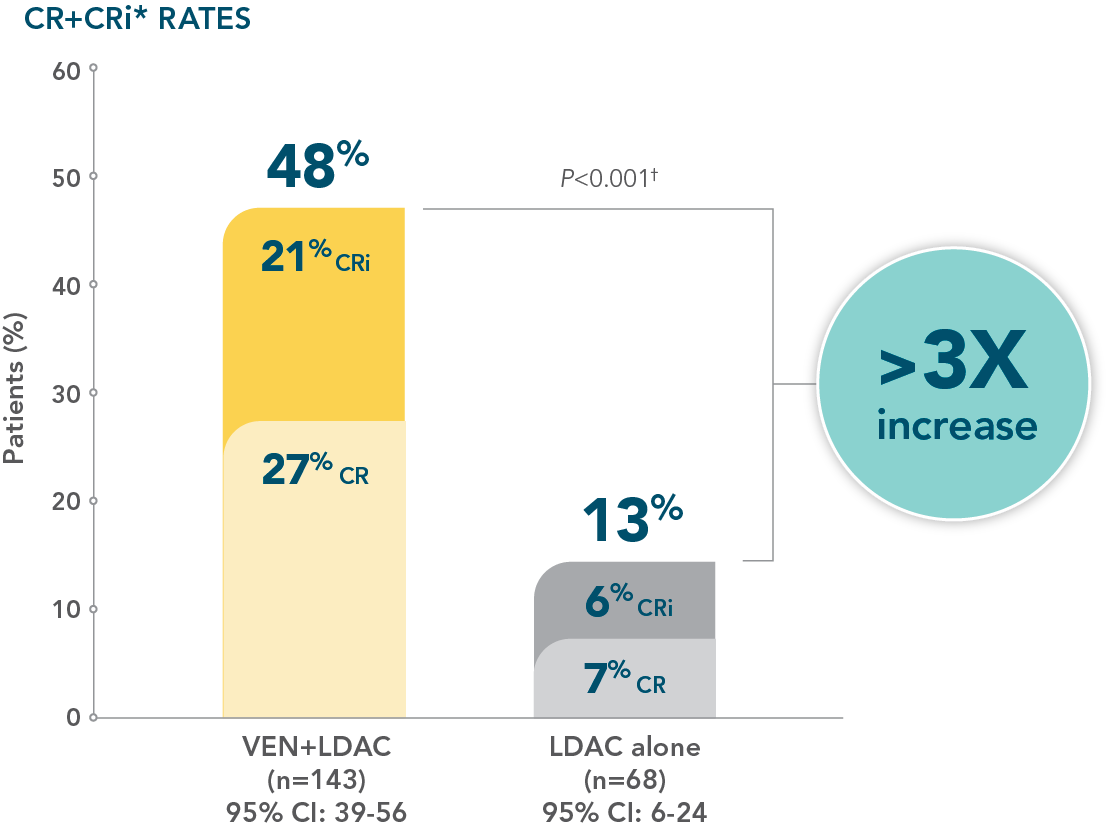
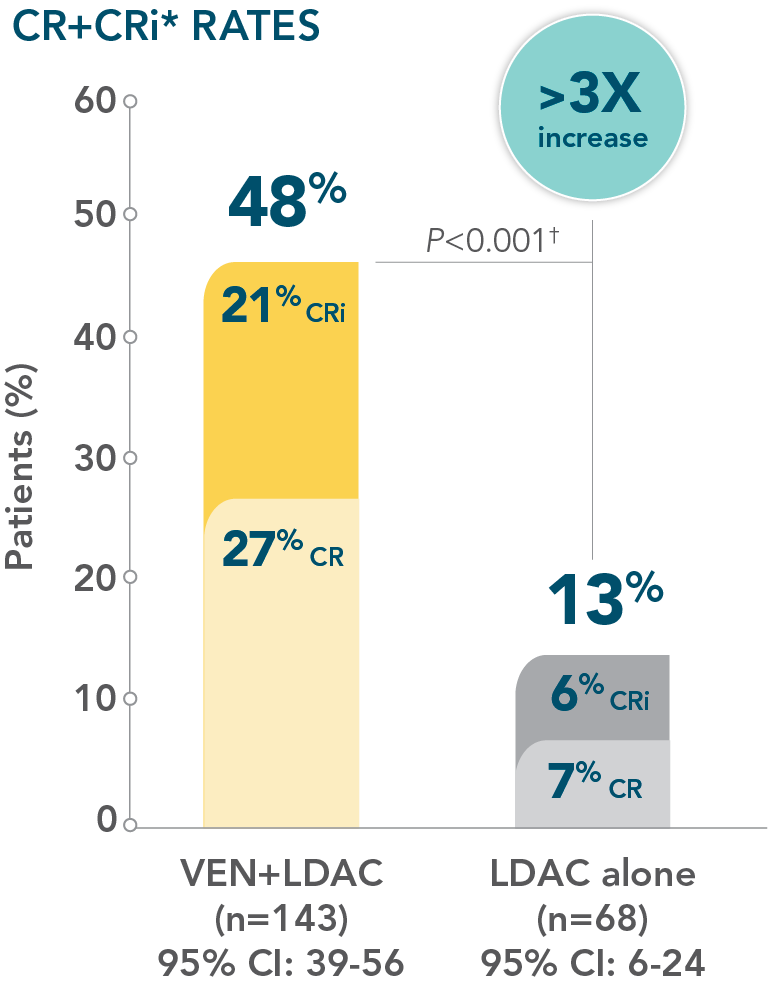
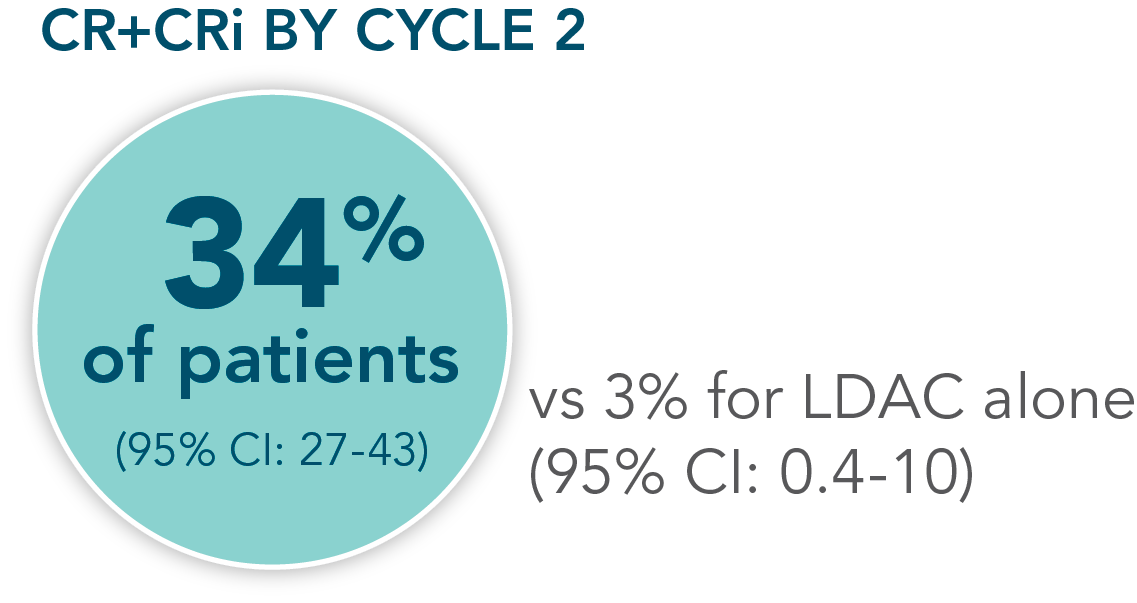
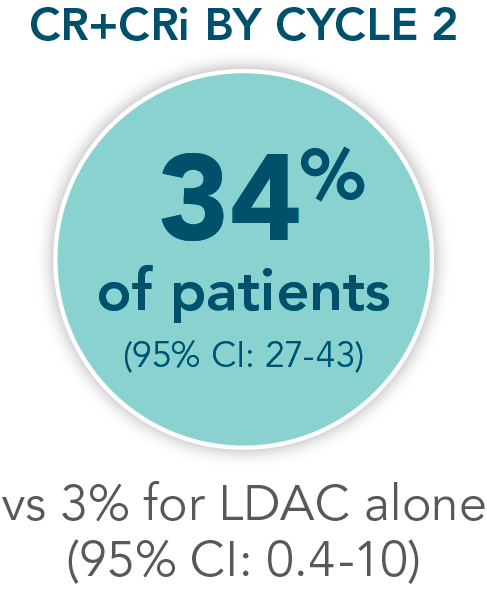
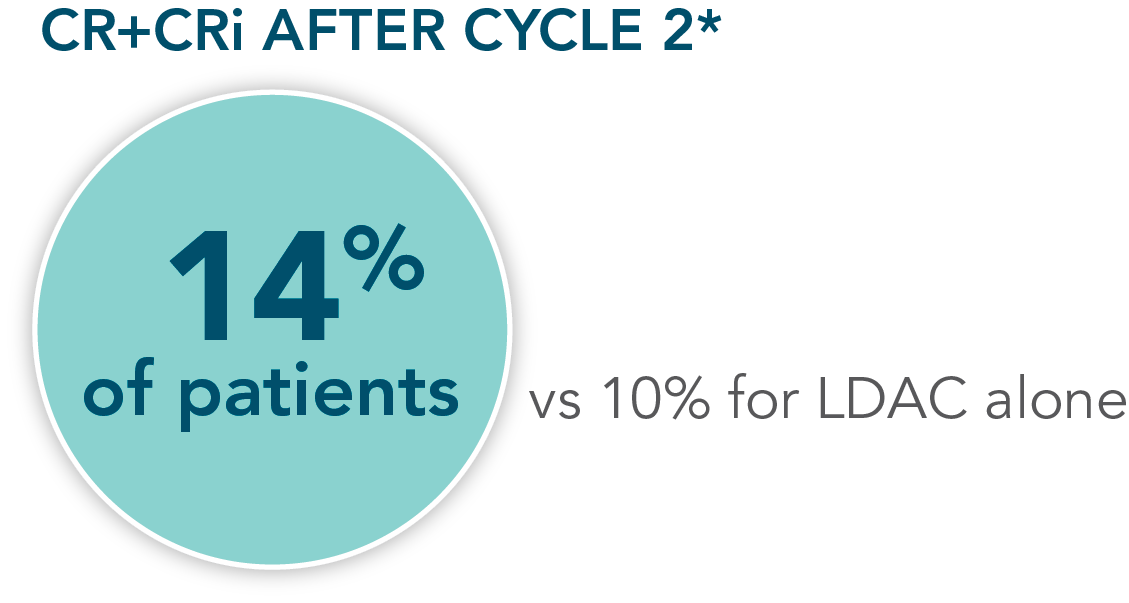
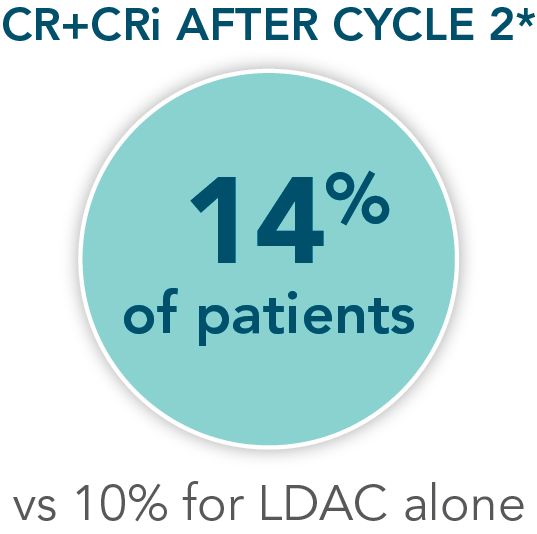
- Increased remission rates with VENCLYXTO plus LDAC vs LDAC alone (48% CR+CRi vs 13%, respectively; P<0.001†)1,2
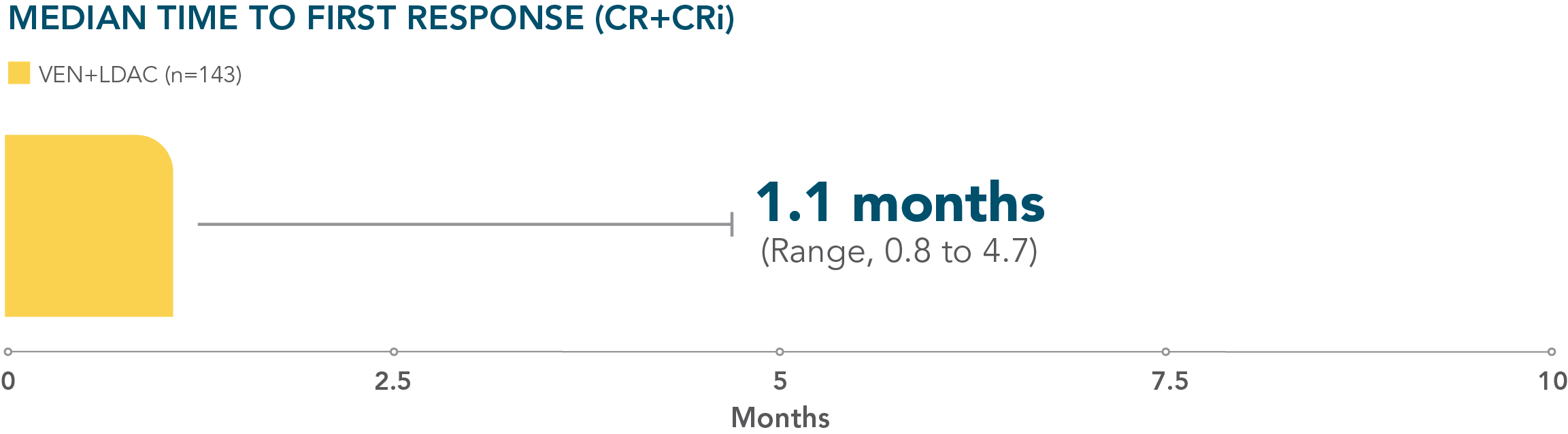
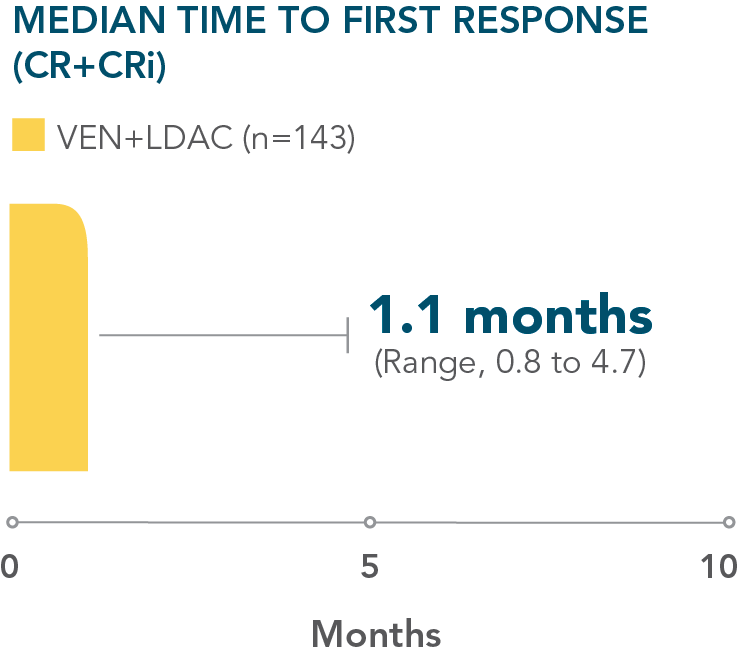
- Patients treated with VENCLYXTO plus LDAC achieved remission in a median of 1.1 months1
Impacted transfusion dependence
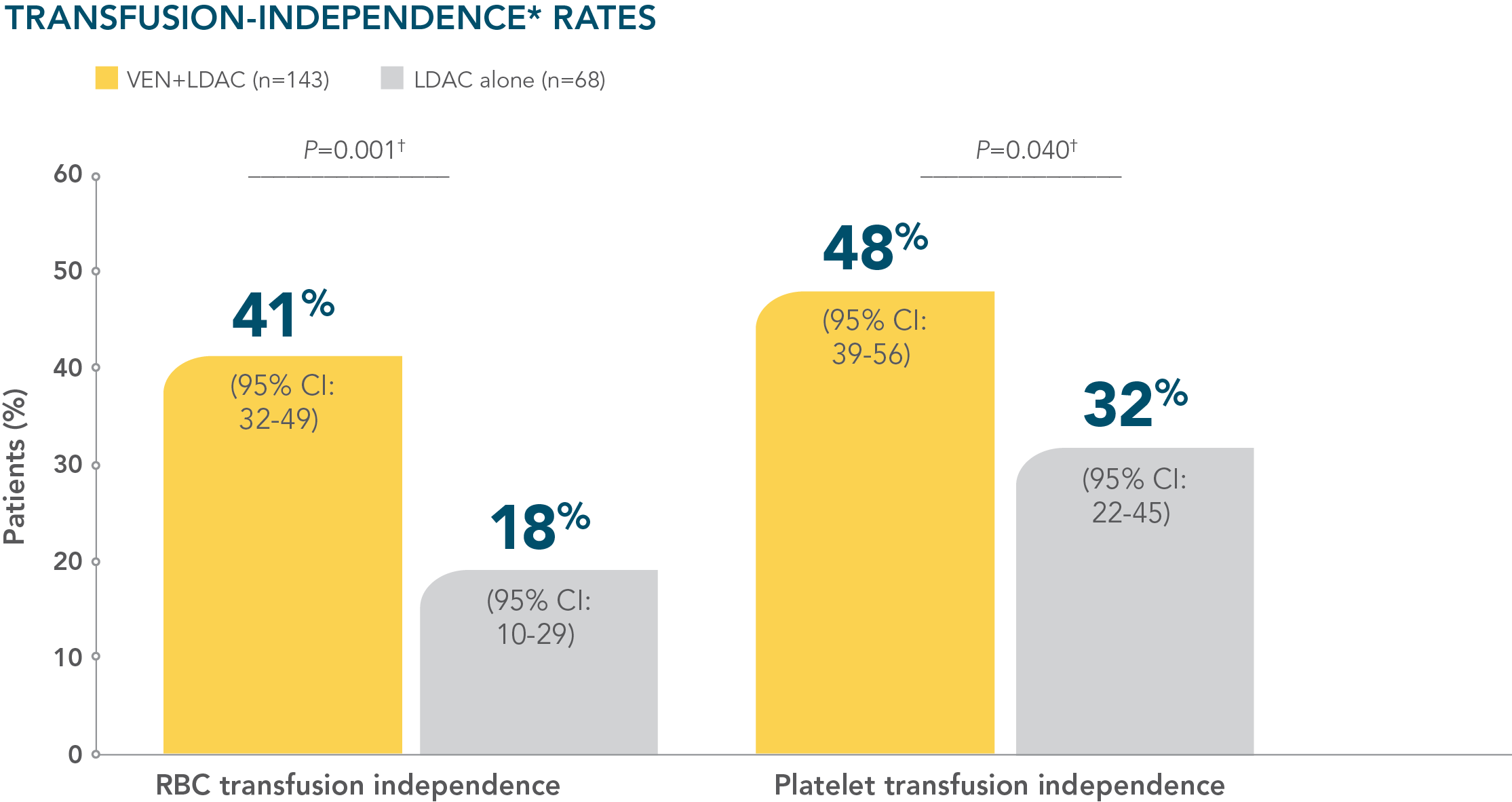
- More than 40% of patients treated with VENCLYXTO plus LDAC achieved independence from both RBC and platelet transfusions1
†This P value is descriptive.
mOS=median overall survival; CR=complete remission; CRi=complete remission with incomplete hematological recovery; RBC=red blood cell.
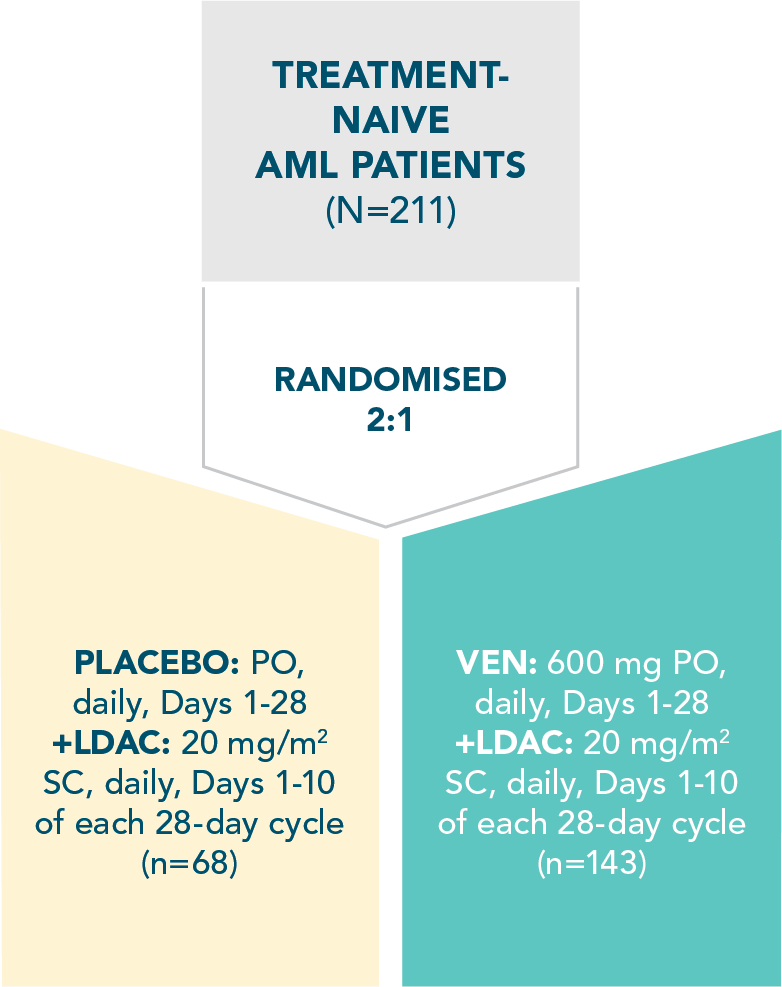
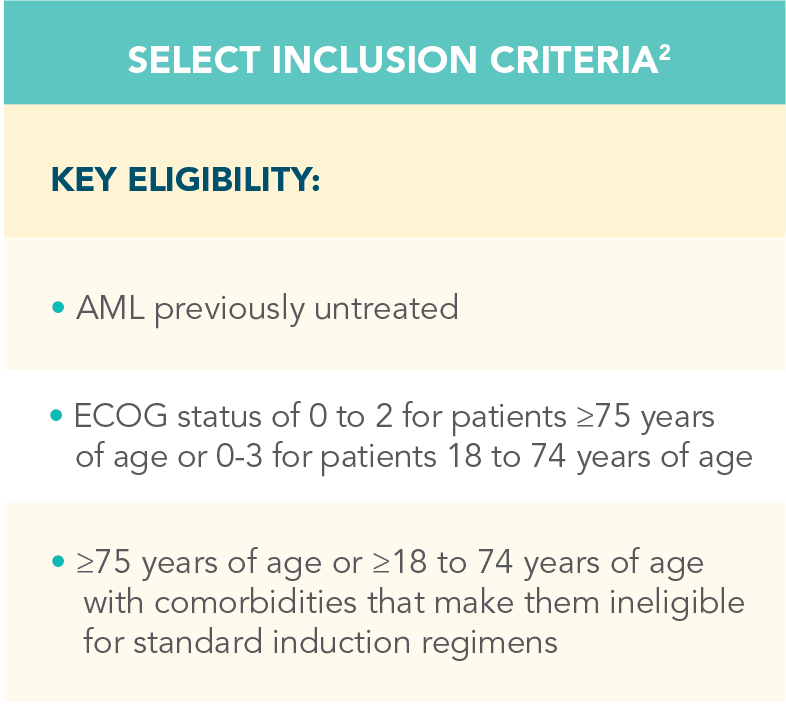
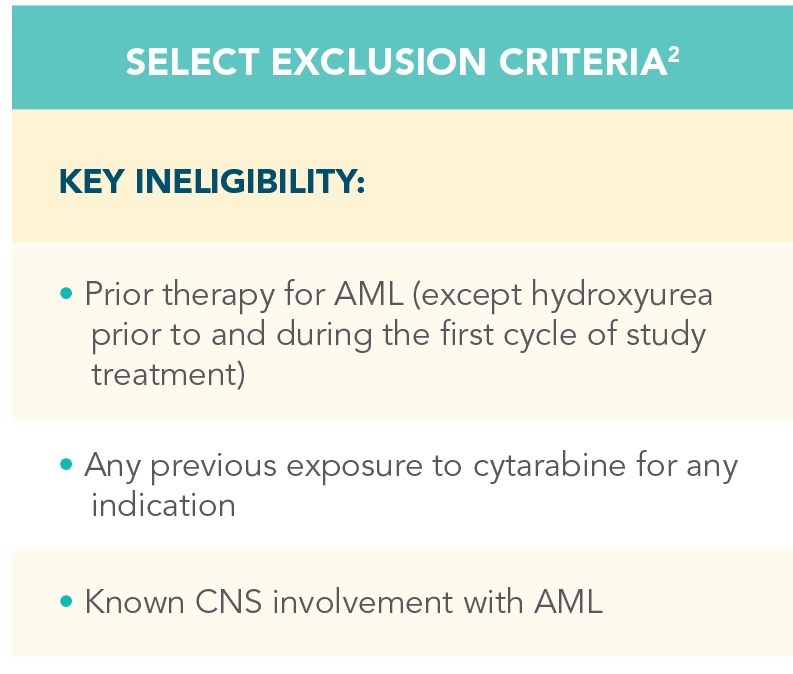
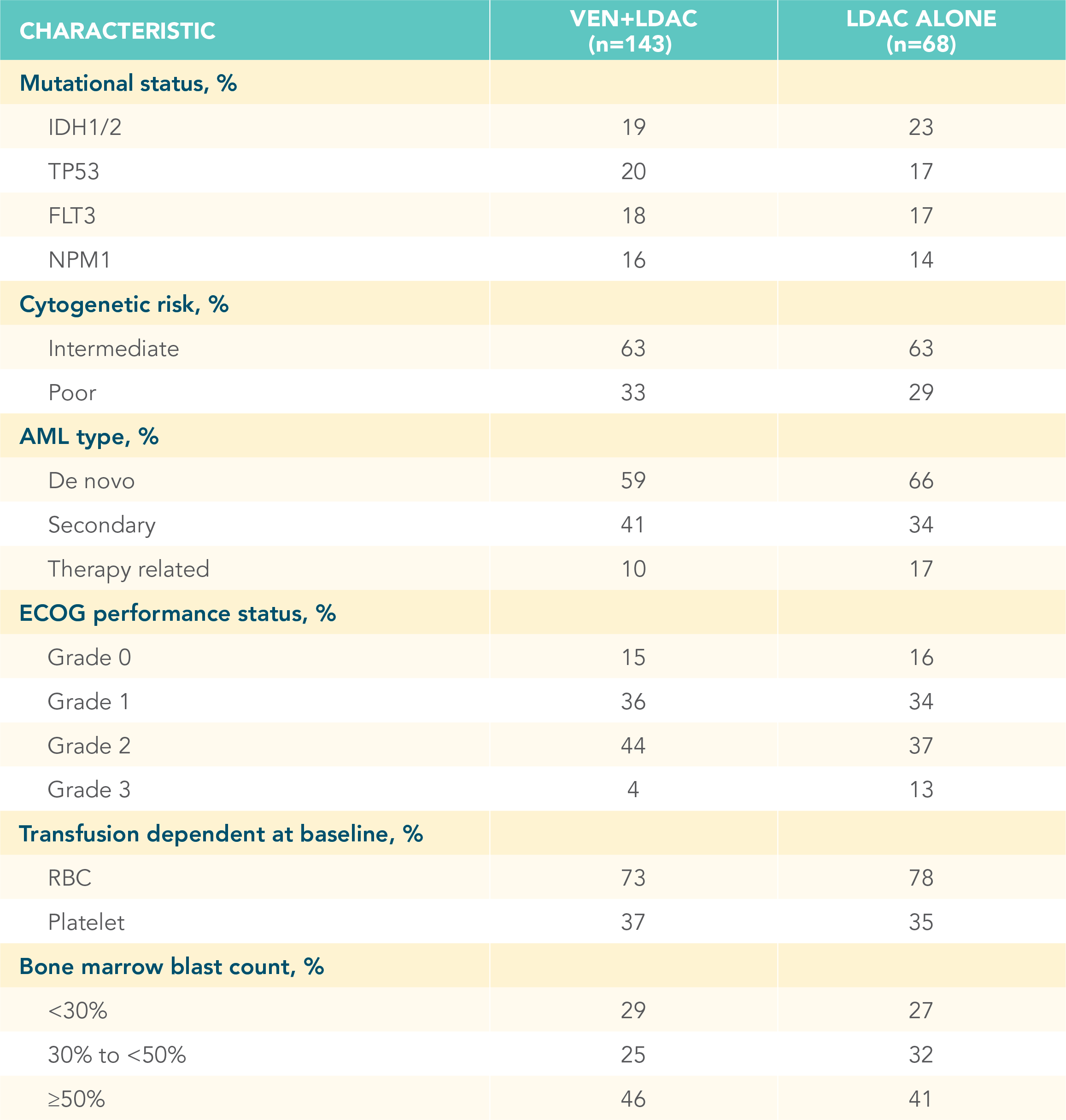
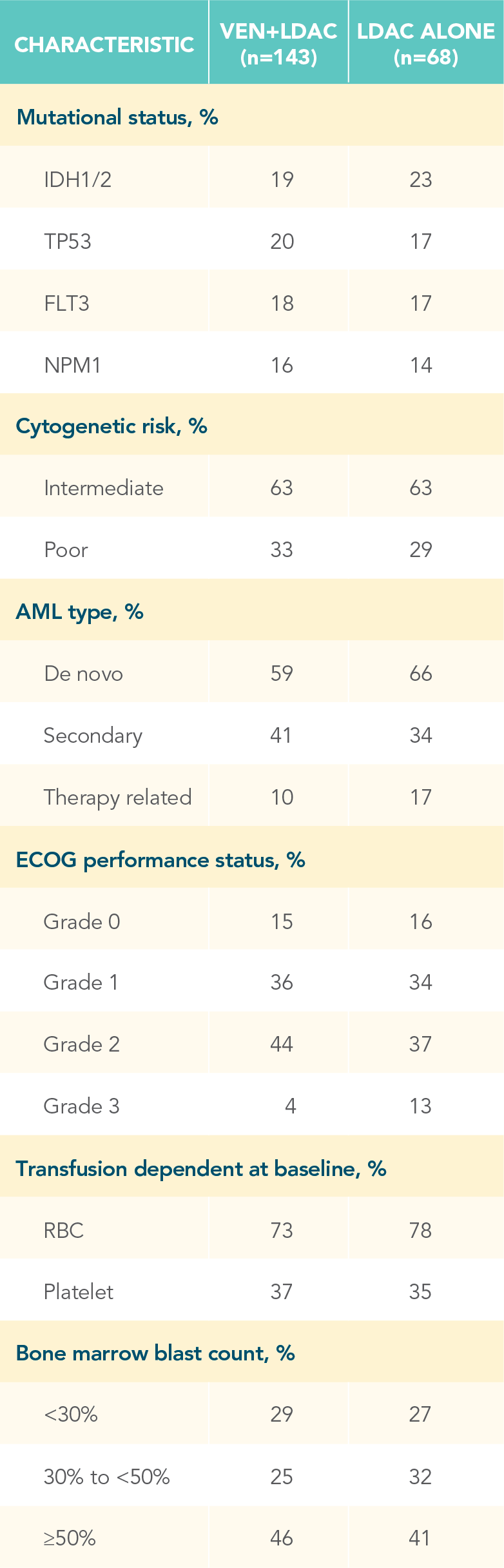
A randomized, double-blind, placebo-controlled study that evaluated the efficacy and safety in patients ineligible for intensive chemotherapy1
*Patients were considered ineligible for intensive chemotherapy if they were 75 years of age or older or had comorbidities that precluded the use of intensive induction chemotherapy.
VEN=VENCLYXTO; PO=by mouth; SC=subcutaneous; ECOG=Eastern Cooperative Oncology Group; CNS=central nervous system.
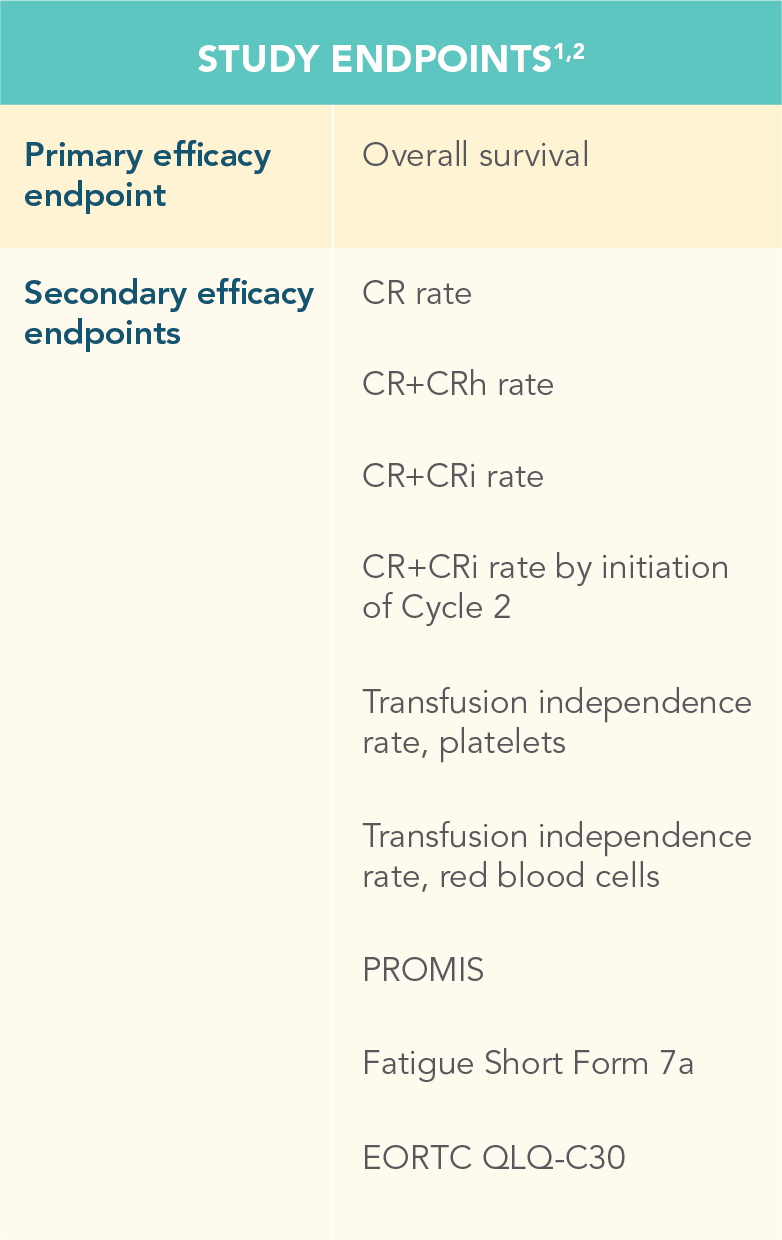
Efficacy and safety were evaluated in newly diagnosed patients with AML
Treatment was continued until disease progression or unacceptable toxicity1
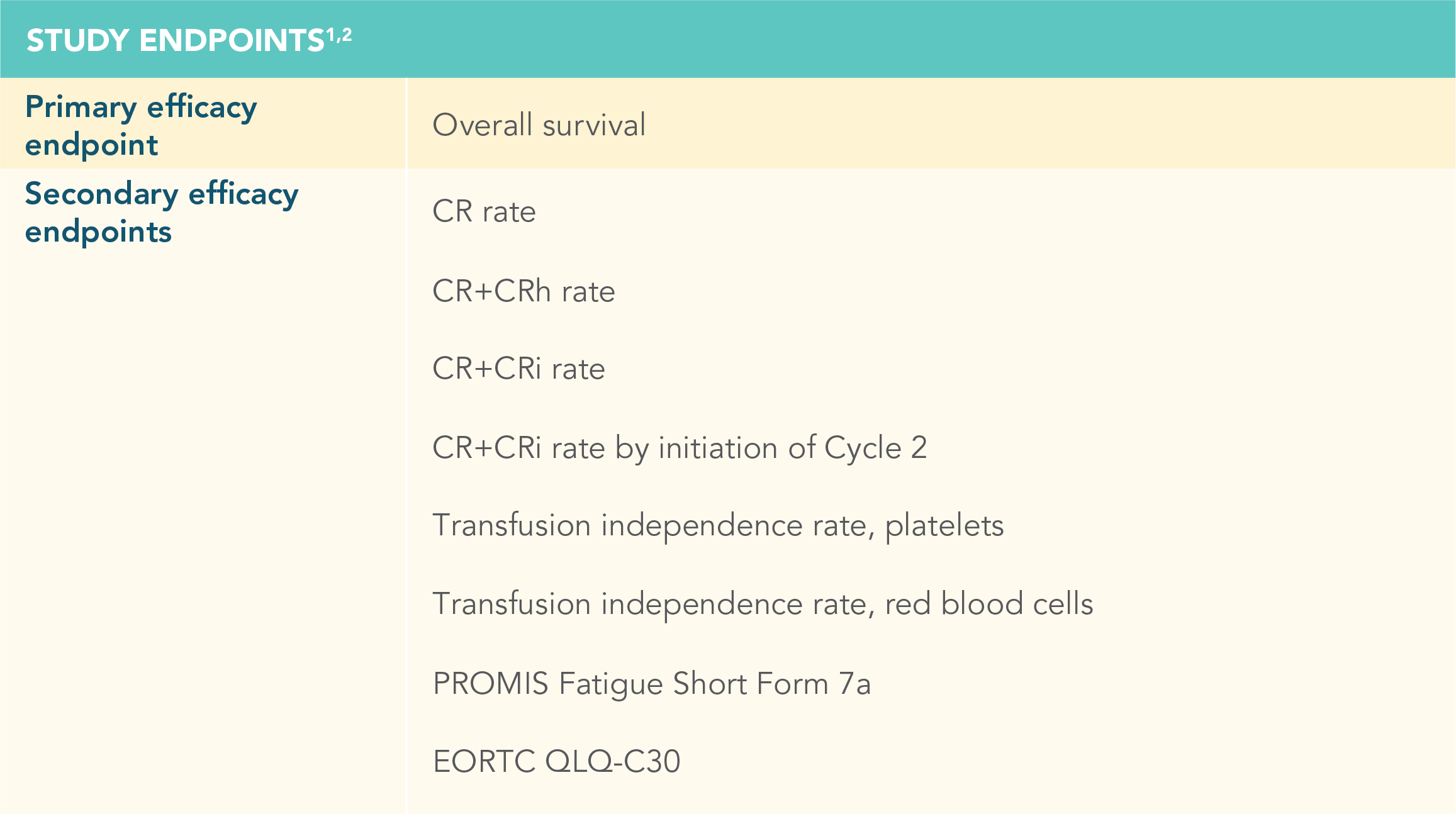
CR=complete remission; CRh=complete remission with partial hematological recovery; PROMIS=Patient-Reported Outcomes Measurement Information System; EORTC QLQ-C30=European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30.
- At the time of primary analysis for overall survival, the median overall survival in the VENCLYXTO plus LDAC group was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07, respectively; P=0.114])1,2
MEDIAN OVERALL SURVIVAL (NOT SIGNIFICANT)1
*“Hard-to-treat patient populations” refers to those patients who are ineligible for intensive chemotherapy.
CI=confidence interval; HR=hazard ratio.
Patients treated with VENCLYXTO plus LDAC experienced a 4.3-month increase in median overall survival vs LDAC alone
*CR=(complete remission) absolute neutrophil count (ANC) >1000/microliter, platelets >100,000/microliter, RBC transfusion independence, and bone marrow with <5% blasts. Absence of circulating blasts and blast with Auer rods, absence of extramedullary disease.1
†This P value is descriptive.
Patients were assessed for remission by conducting a bone marrow test at the end of Cycle 1 and during treatment as needed1
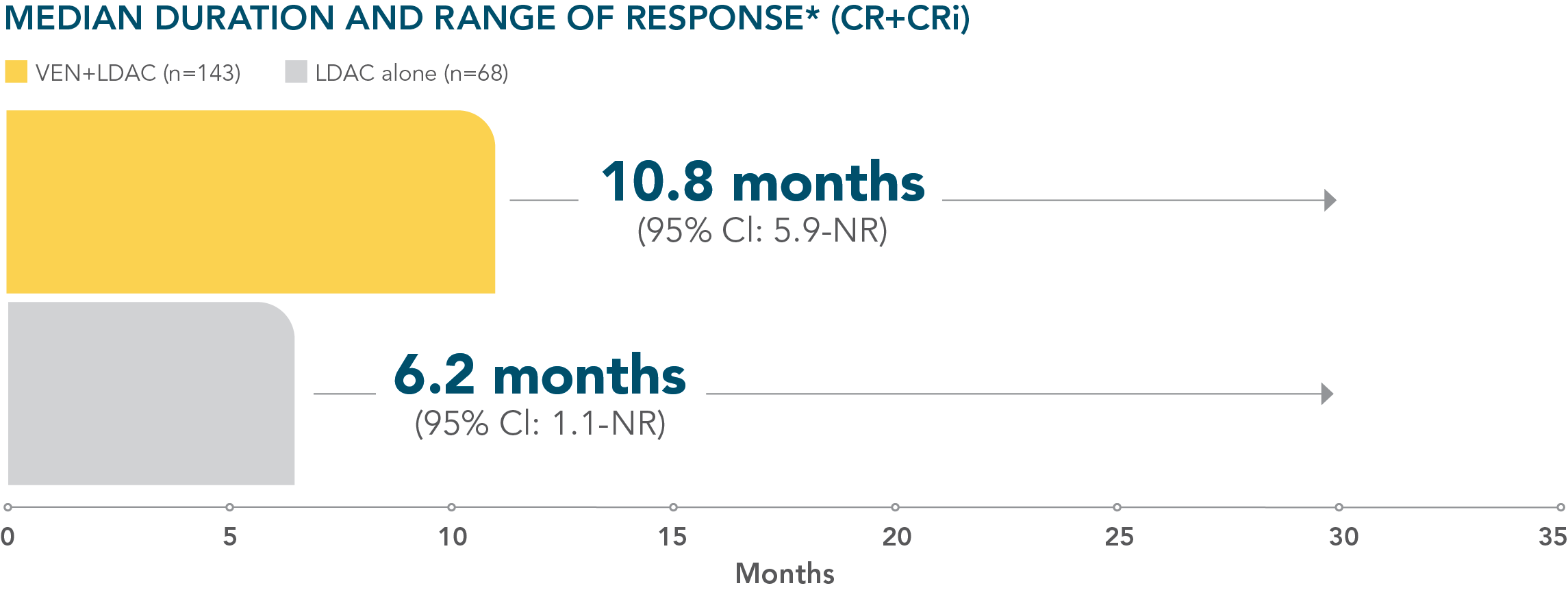
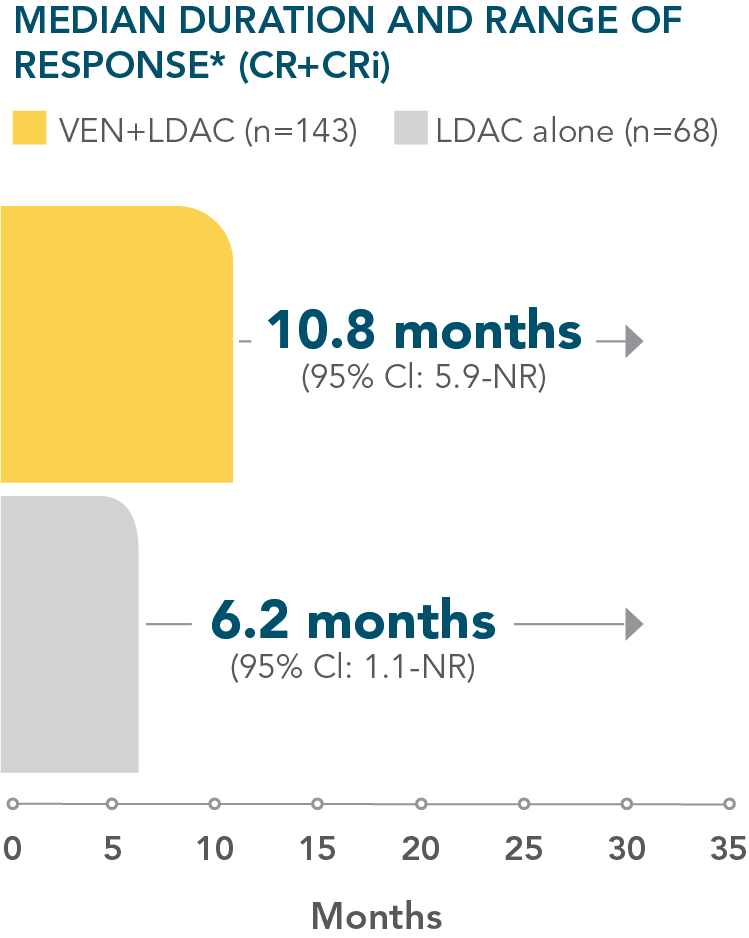
*Median duration of response is from Kaplan-Meier estimate and was defined as time from first response of CR or CRi to the first date of confirmed morphologic relapse, confirmed progressive disease, or death due to disease progression, whichever occurred earlier.1
NR=not reported.
Continue treatment until disease progression or unacceptable toxicity1
More than 40% of patients treated with VENCLYXTO plus LDAC achieved independence from both RBC and platelet transfusions1,2
*Transfusion independence was defined as a period of at least 56 consecutive days with no transfusion after the first dose of study drug and on or before the last dose of the study drug plus 30 days, or before relapse or disease progression, or before the initiation of posttreatment therapy, whichever is earlier.1
†This P value is descriptive.
I want to find out more
about VENCLYXTO
I want to receive more information about VENCLYXTO
References: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. <Current SmPC.> 2. Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy: phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145.