For newly diagnosed patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy1
VENCLYXTO is the first approved BCL-2 inhibitor for the treatment of AML1
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
*VIALE-A was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus AZA in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus AZA was 14.7 months (95% CI: 11.9-18.7) vs 9.6 months for AZA alone (95% CI: 7.4-12.7) (HR=0.66 [95% CI: 0.52-0.85; P<0.001]).1
†VIALE-C was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus LDAC was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07; P=0.114]).1
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
BCL-2=B-cell lymphoma 2; AZA=azacitidine; LDAC=low-dose cytarabine; CI=confidence interval; HR=hazard ratio.
For newly diagnosed patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy1
VENCLYXTO is the first approved BCL-2 inhibitor for the treatment of AML1
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
VENCLYXTO plus azacitidine demonstrated a 5.1-month increase in median overall survival vs AZA alone1*
VENCLYXTO plus low-dose cytarabine showed a 3.1-month increase in median overall survival vs LDAC alone1†
*VIALE-A was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus AZA in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus AZA was 14.7 months (95% CI: 11.9-18.7) vs 9.6 months for AZA alone (95% CI: 7.4-12.7) (HR=0.66 [95% CI: 0.52-0.85; P<0.001]).1
†VIALE-C was a randomized (2:1), double-blind, placebo-controlled, phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus LDAC was 7.2 months (95% CI: 5.6-10.1) vs 4.1 months for LDAC alone (95% CI: 3.1-8.8) (HR=0.75 [95% CI: 0.52-1.07; P=0.114]).1
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
▼
BCL-2=B-cell lymphoma 2; AZA=azacitidine; LDAC=low-dose cytarabine; CI=confidence interval; HR=hazard ratio.
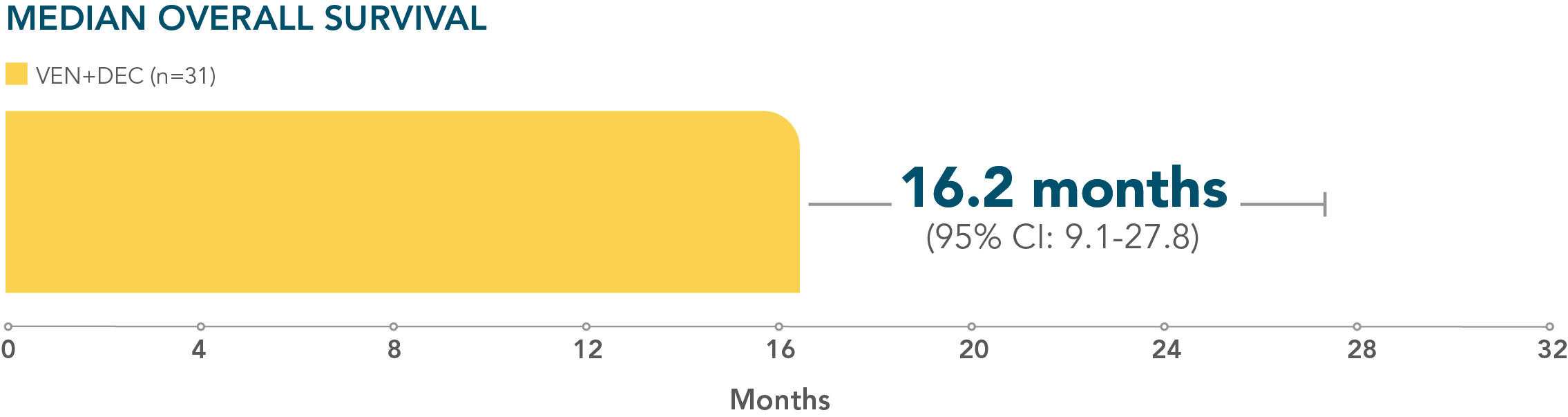
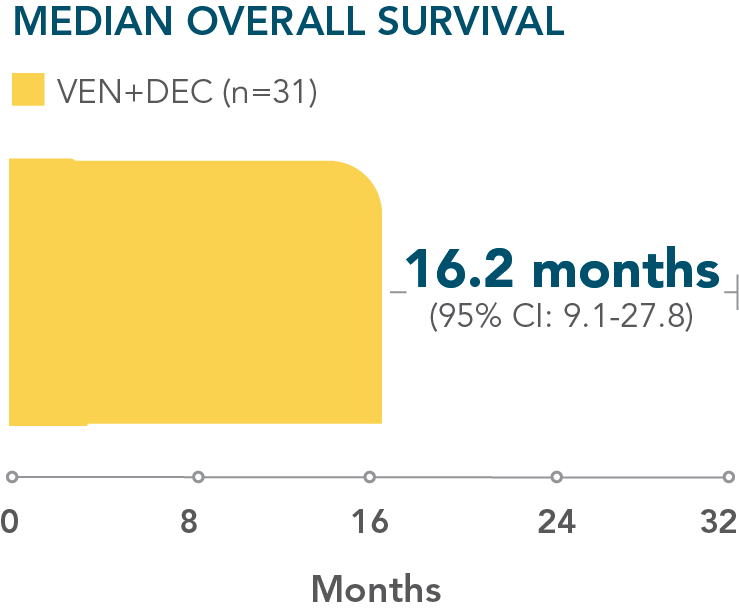
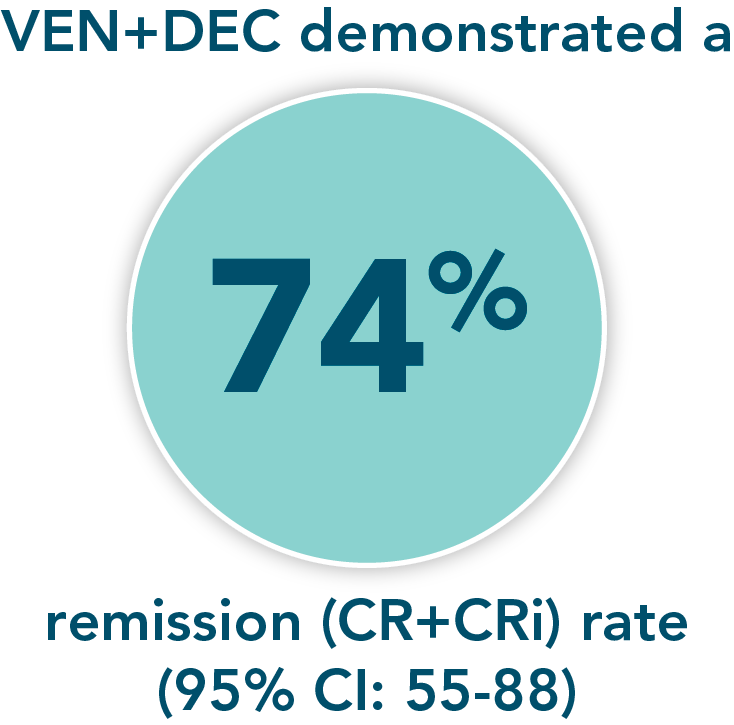
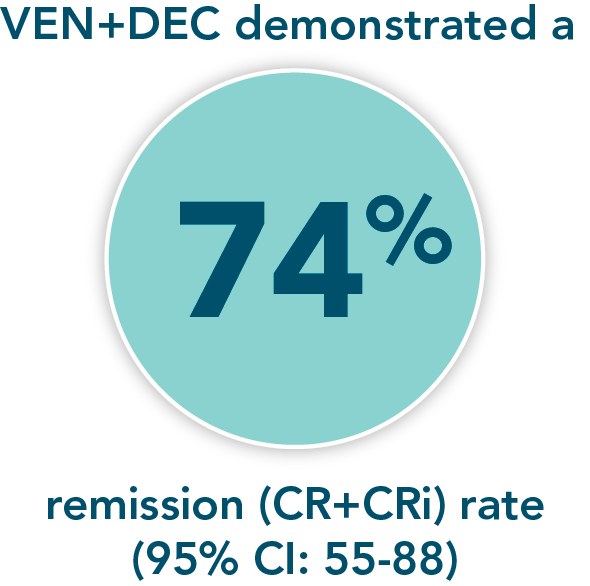
*M14-358 was a nonrandomized, phase 1/2 trial evaluating the safety and efficacy of VENCLYXTO + decitabine in patients with newly diagnosed AML who were ineligible for intensive chemotherapy.
- Median overall survival with VEN+DEC: 16.2 months (95% CI: 9.1–27.8; n=31)
- Remission rate (CR+CRi) with VEN+DEC: 74% (95% CI: 55-88)
VEN=VENCLYXTO; DEC=decitabine; CR=complete remission; CRi=complete remission with incomplete hematological recovery.
A nonrandomized study in patients with newly diagnosed AML who were ineligible for intensive chemotherapy1
- Patients received VENCLYXTO via a daily titration to a final daily dose of 400 mg. DEC 20 mg/m2 was administered intravenously on Days 1-5 of each 28-day cycle, beginning on Cycle 1 Day 1
- The median age of patients treated with VENCLYXTO plus DEC was 72 years (range: 65-86 years); 87% were white; 48% were male, and 87% had an ECOG score of 0 or 1
- The median duration of follow-up was 40.4 months (range: 0.7 to 42.7 months)
ECOG=Eastern Cooperative Oncology Group.
I want to find out more
about VENCLYXTO
I want to receive more information about VENCLYXTO
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. <Current SmPC.>