Note to affiliates: This update to the venetoclax CLL AbbVie Pro site includes a homepage headline, updated CLL14 6-year, CLL13 4-year, MURANO 7-year data sets, and other streamlined content updates. CLL 13 4-year update reflects the CLL13 data from the Lancet Oncology publication. The CLL14 6-year and MURANO 7-year data have been updated based on the EHA 2023 abstracts. For countries that cannot use these data sets, please follow local regulations and MRLO guidance, and revert to CLL14 5-year and MURANO 5-year published data from the product label.
Primary analysis in ITT population for VEN+O vs O+Clb1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.35; 95% CI: 0.23–0.53 [P<0.0001]).
| • | Median follow-up of 28 months |
Additional analyses:
6-year PFS estimate (INV-assessed)2‡: 53% vs 22% (HR=0.40; 95% CI: 0.31–0.52) after 5 years off treatment.
| • | Median PFS of 76.2 months with VEN+O vs 36.4 months with O+Clb |
INV-assessed complete remission (CR/CRi)1: 50% vs 23% (P<0.0001).
| • | ORR: 85% (95% CI: 79.2–89.2) vs 71% (95% CI: 64.8–77.2 [P=0.0007]) |
Primary analysis in ITT population for VEN+R vs BR1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.17; 95% CI: 0.11–0.25 [P<0.0001]).
| • | Median follow-up of 23.8 months |
Additional analyses:
7-year PFS estimate (INV-assessed)3‡: 23% (HR=0.23; 95% CI: 0.18–0.29) vs NE after ~5 years off treatment.
| • | Median PFS of 54.7 months with VEN+R (95% CI: 52.3–59.9) vs 17.0 months with BR (95% CI: 15.5–21.7) |
INV-assessed complete remission (CR/CRi)1‡: 27% vs 8%.
| • | ORR: 93% (95% CI: 88.8–96.4) vs 68% (95% CI: 60.6–74.2) |
*See full dosing information for VEN+O and for VEN+R in the dosing and administration section.
†Primary endpoint.
‡Results are descriptive only.
1L=first line; CLL=chronic lymphocytic leukaemia; VEN+O=VENCLYXTO + obinutuzumab; ITT=intent to treat; O+Clb=obinutuzumab + chlorambucil; INV=investigator; PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete bone marrow recovery; ORR=overall response rate; 2L+=second line + later lines of therapy; VEN+R=VENCLYXTO + rituximab; BR=bendamustine + rituximab; NE=not evaluable.
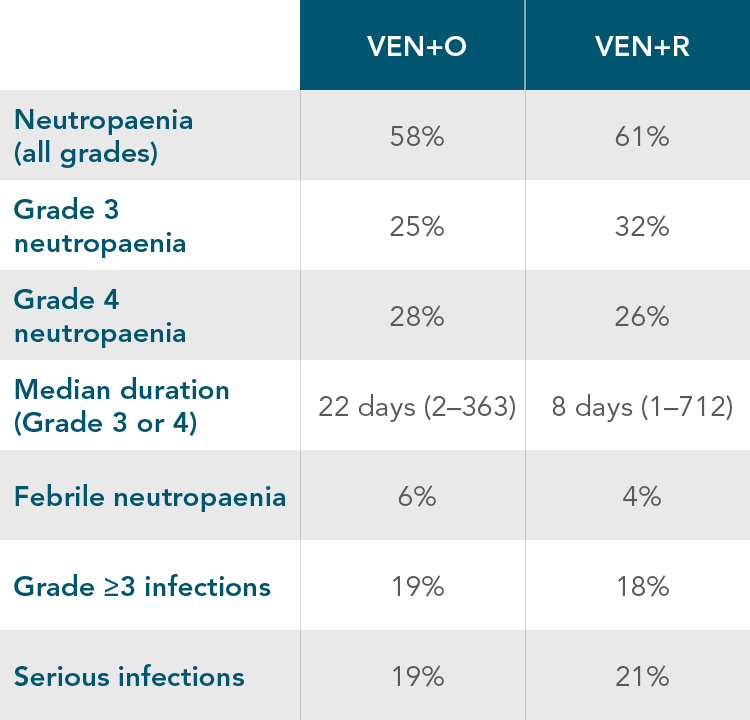
NEUTROPAENIA AND INFECTIONS1
Grade 3 or 4 neutropaenia has been reported in patients treated with VENCLYXTO in combination studies with rituximab or obinutuzumab and in monotherapy studies. Complete blood counts should be monitored throughout the treatment period. Dose interruptions or reductions are recommended for patients with severe neutropaenia.
Serious infections, including sepsis with fatal outcome, have been reported. Monitoring of any signs and symptoms of infection is required. Suspected infections are to receive prompt treatment, including antimicrobials and dose interruption or reduction as appropriate.
ADDITIONAL INFORMATION—NEUTROPAENIA AND INFECTIONS
1L=first line; 2L+=second line + later lines of therapy; BR=bendamustine + rituximab; CLL=chronic lymphocytic leukaemia; CR=complete remission; CRi=complete remission with incomplete bone marrow recovery; CI=confidence interval; EoCT=end of combination treatment; EoT=end of treatment; HR=hazard ratio; ITT=intent to treat; INV=investigator; MRD=minimal residual disease; nPR=nodular partial remission; O+Clb=obinutuzumab + chlorambucil; ORR=overall response rate (CR+CRi+PR); PR=partial remission; PFS=progression-free survival; PB=peripheral blood; VEN+O=VENCLYXTO + obinutuzumab; VEN+R=VENCYLXTO + rituximab.
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. <Current SmPC.>