VIALE-C evaluated VENCLYXTO plus low-dose cytarabine (VEN+LDAC), an alternative to combination with hypomethylating agents (VEN+HMAs). The primary endpoint (statistically significant improvement in overall survival) was not met, therefore, secondary endpoints are descriptive only. VIALE-C was a randomised (2:1), double-blind, placebo-controlled, Phase 3 study that evaluated the efficacy and safety of VEN+LDAC in patients with newly diagnosed AML who were ineligible for intensive chemotherapy.1,2
SAFETY/TOLERABILITY FOR VENCLYXTO + LOW-DOSE CYTARABINE
DISCONTINUATION RATES WITH VENCLYXTO PLUS LDAC
DISCONTINUATIONS, DOSE INTERRUPTIONS, AND DOSE REDUCTIONS DUE TO AEs FOR VEN+LDAC VS LDAC ALONE, RESPECTIVELY1,2
| • | 25% of patients discontinued treatment vs 24% |
| • | 63% of patients experienced dose interruptions vs 53% |
| • | 9% of patients had dose reductions vs 6% |
| • | The most common AEs that led to dose reductions or interruptions were neutropaenia, thrombocytopaenia, pneumonia, febrile neutropaenia, and sepsis (excluding fungal) |
VENCLYXTO PLUS LDAC SAFETY AND TOLERABILITY PROFILE
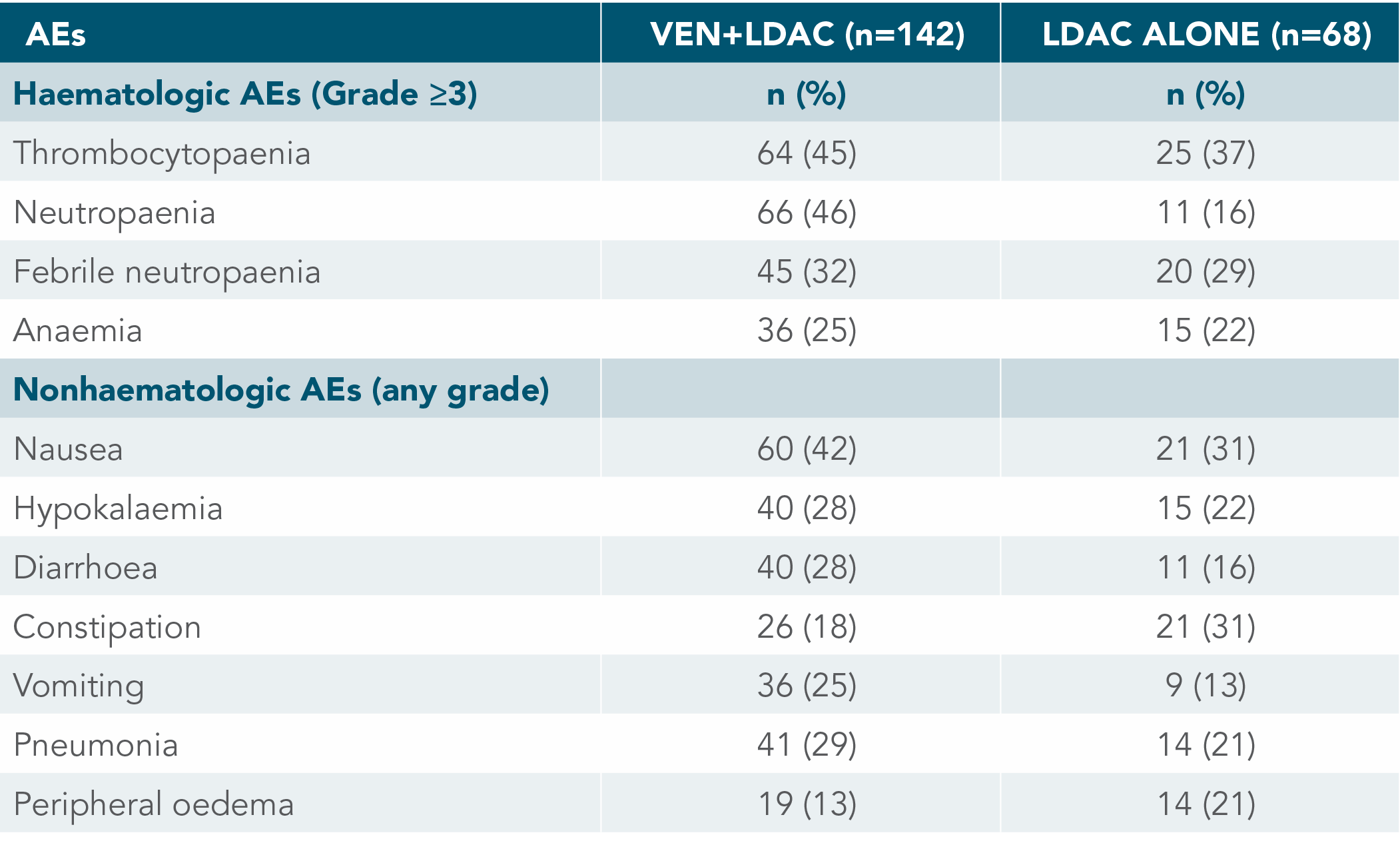
Adverse events with VENCLYXTO plus LDAC were manageable and well-characterised1,2
MOST COMMON TREATMENT-EMERGENT AEs (≥20% IN EITHER TREATMENT ARM)
SERIOUS TREATMENT-EMERGENT AEs (ANY GRADE)
| • | Of the patients treated with VENCLYXTO plus LDAC, 8 (5.6%) experienced TLS, including those who died or experienced renal failure1,2 *Aligns with VENCLEXTA US Prescribing Information. |
VENCLYXTO + LDAC ADVERSE REACTIONS
ADVERSE REACTIONS (≥10%) IN PATIENTS WITH AML WHO RECEIVED VEN+LDAC IN VIALE-C STUDY1*
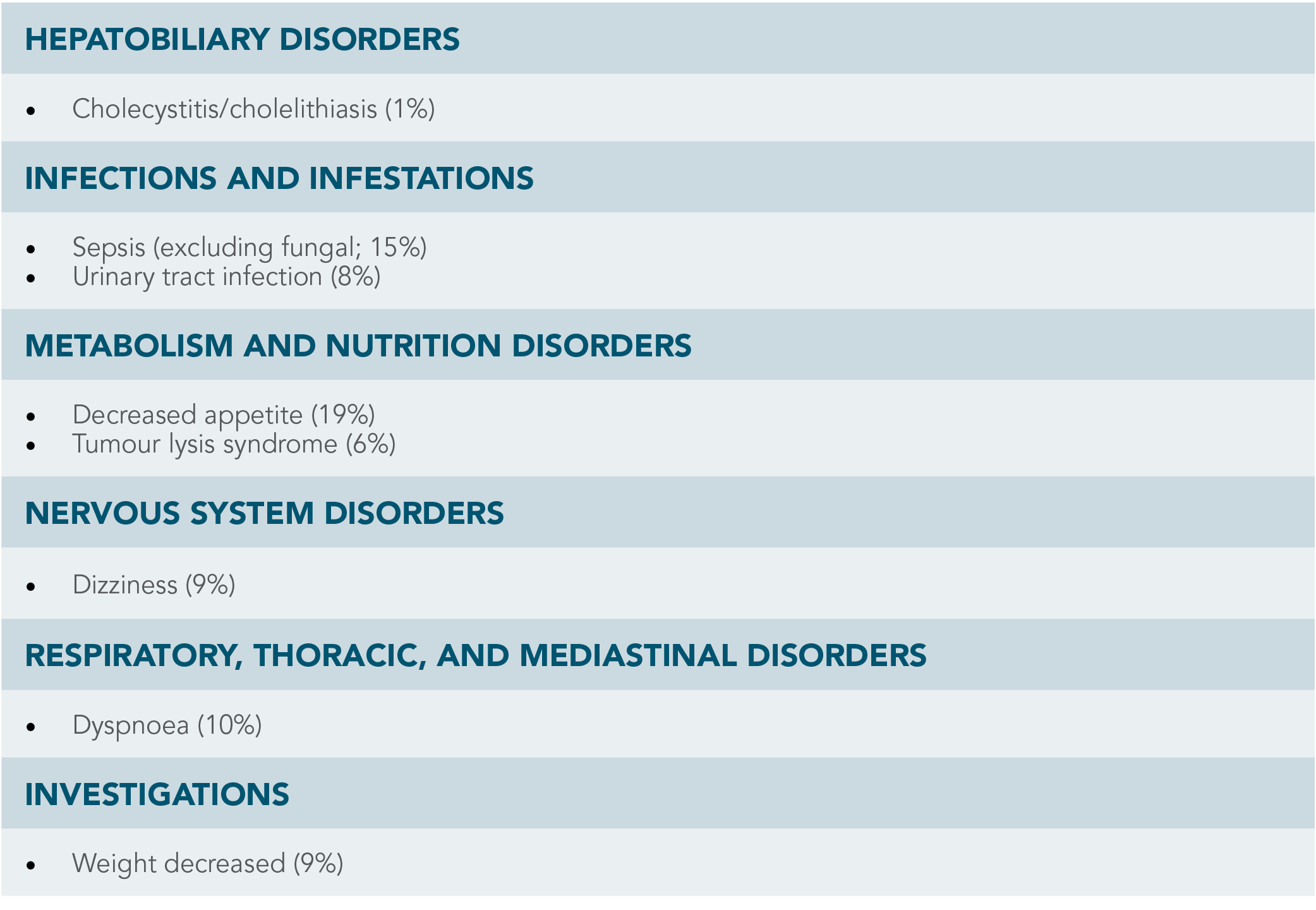
OTHER CLINICALLY IMPORTANT ADVERSE REACTIONS1
*Includes patients with a difference between arms of ≥5% for all grades or ≥2% for Grade 3 or 4 compared with placebo+LDAC.
†Includes other terms that can be found on VENCLEXTA US Prescribing Information.
AML=acute myeloid leukaemia; AE=adverse event; BCL-2=B-cell lymphoma 2; HMA=hypomethylating agent; LDAC=low-dose cytarabine; TLS=tumour lysis syndrome; VEN=VENCLYXTO.
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
References: 1. VENCLEXTA Prescribing Information. North Chicago, IL: AbbVie Inc. 2. Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
ALL-VNCAML-220066 March 2023