Chronic migraine management at the interface between primary and secondary care
The content below is extracted from the NHS Getting It Right First Time review of neurology.6
The full report can be accessed here
Most patients with headache have either migraine or another primary headache disorder. Many can self-manage their headaches or manage them with support from their GPs.
Help for GPs to begin appropriate management and avoid unnecessary referral includes:
- diagnosis and management guidelines (eg NICE, BASH, NHS)
- site-specific locally applicable guidance
- referral assessment advice
Development of an easily accessible local headache pathway may improve headache management.
“The challenge for commissioners is that most headaches referred to secondary care end up with a diagnosis of migraine and/or medication overuse headache, which is best managed in the community, which is easier for patients to access and cheaper for the healthcare economy”
NHS RightCare7
Using the standard ‘choose and book’ electronic referral service (e-RS), patients who have been referred by the GP will have selected their appointment time before the neurologist is able to review and assess the referral letter. While convenient for patients, this method limits the flexibility neurologists have to manage each referral (eg by providing direct advice or redirecting to a more specialist clinic). One solution is to triage outpatient referrals using the Referral Assessment Service within the NHS e-RS.
GIRFT Recommendation 4
Implement advice and guidance and a triaging system of outpatient referrals to ensure effective management of referrals, offer earlier management advice, improve clinic waiting times and reduce DNAs.
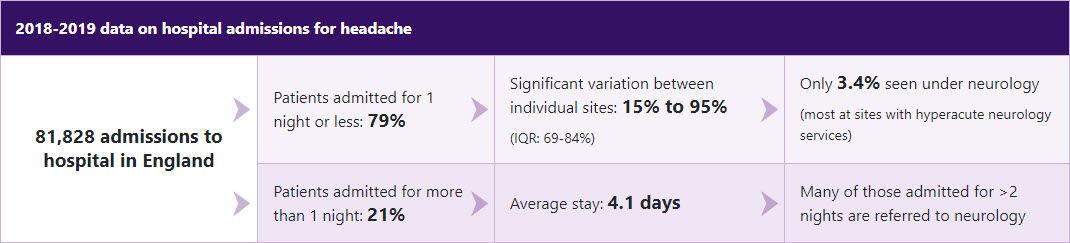
Most patients who attend hospital for headache are managed in A&E or in an acute medical unit.
On average, 9% of patients are readmitted with headache within 12 months. There is considerable variation in this readmission rate between sites.
GIRFT Recommendation 16
Ensure regular review of readmission rates for headaches to understand and address variation, to ensure the pathway for these patients is optimised.
(a) Review readmission rates for headache and carry out local audit of any high rates
BASH: British Association for the Study of Headache; CCGs: clinical commissioning groups; CM: chronic migraine; DNA: ‘did not attend’ appointment; IQR: interquartile range; NICE: National Institute for Health and Care Excellence.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: March 2024. UK-BCM-240048