This promotional material is intended for UK Healthcare Professionals only. BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
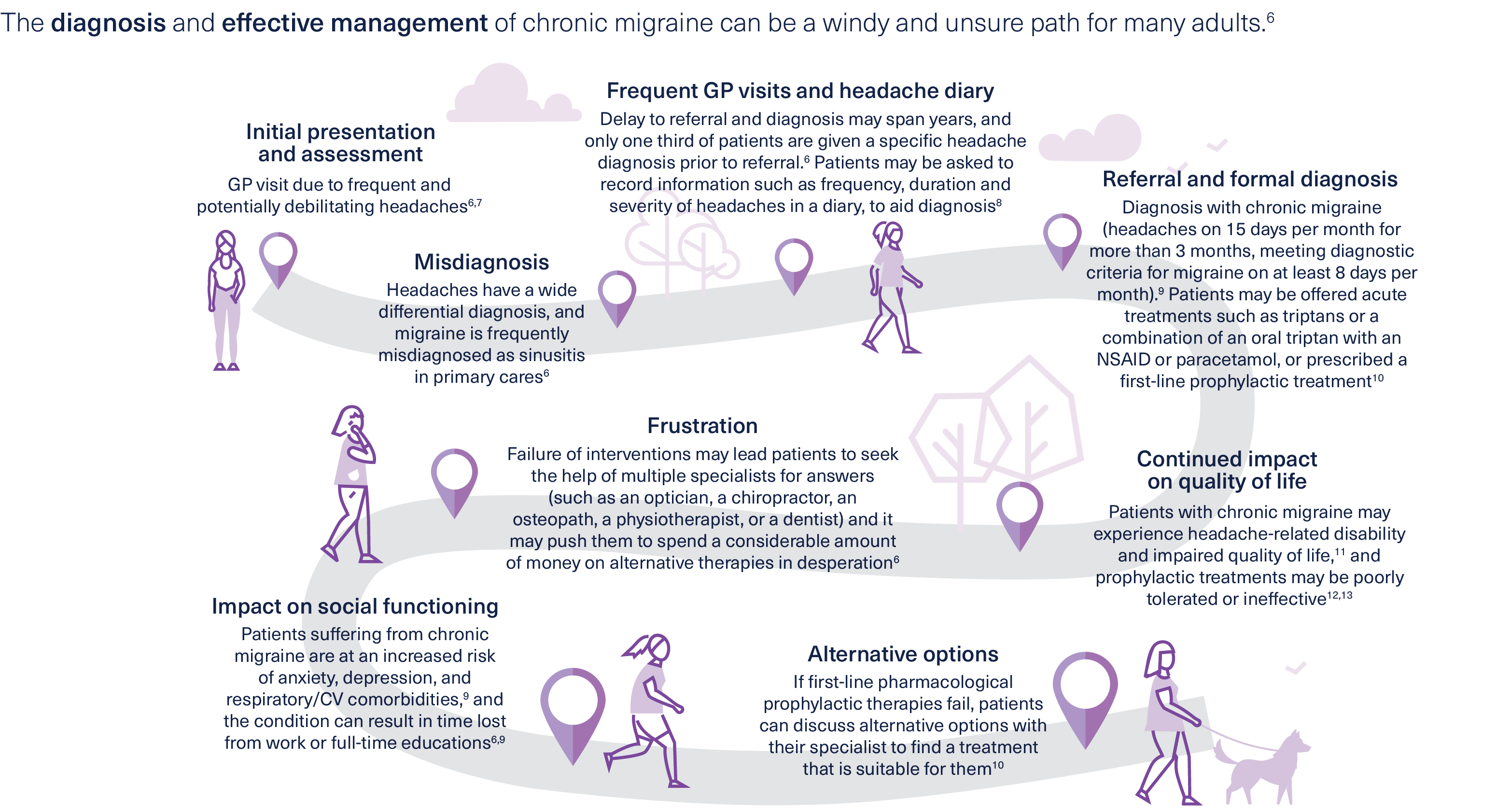
Diagnosing and managing chronic migraine
Could your patients with chronic migraine benefit from treatment with BOTOX®?
A real-life patient treatment journey
Kayte was diagnosed with chronic migraine at 24, and experienced issues with certain treatments before finding one that suited her.
Learn about her individual experience in this video, and find out about her experience with BOTOX® >
Initiating a patient on BOTOX®
How should you consult your patients who are starting on BOTOX®, and how can you ensure treatment adherence?
Neurologist Dr Andrew Blumenfeld explains in this 6-minute video >
CM: chronic migraine; CV: cardiovascular; ICHD: International Classification of Headache Disorders.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BCM-250064.