Neuroscience/
Neuroplasticity
Clinical Data
• Marie is a 35-year-old woman who has been referred to your care by her physician
• Let’s hear from Marie to find out more about why she has come to see you today
This is a hypothetical BOTOX®-treated patient case study developed in collaboration with an expert based on their experience with many patients. Case studies are intended to encourage scientific debate and exchange and to enhance appropriate patient management. Case studies are representative only, and individual patient responses may vary.
Lifestyle and Assessment
Decision Point:
Goal Setting and Patient Assessment
You have seen Marie’s clinical presentation, she is able to pick up objects but has difficulty releasing them. Based on the clinical results shown on the previous slide, a BOTOX® injection session was scheduled.1
Goal Setting
Marie has been set the following goal to achieve through her treatment.
The rationale for this decision is below.
Decision Point:
Upper Limb Assessment
You have taken a look at Marie’s current profile and the goal set to be achieved for her treatment.
Ashworth and Tardieu Scales
Finochietto Test
Box and Block Test
Jebsen-Taylor Hand Function Test
Video Recording
3D Movement Analysis
Other
*Recommended assessment measures may vary by country. Please refer to your local guidance on upper limb spasticity for further information on recommended assessments.
You have received the results of all the assessments that were performed on Marie.
Ashworth Scale and Tardieu Scale
Spasticity was assessed using the Ashworth Scale (reference international scale) and the Tardieu Scale (gives the ‘spasticity angle’ and the ‘paresis angle’, which are more sensitive to change). Spasticity was graded as follows:
• Wrist Flexors: A1 T1 130/90/120 (neutral position is 90°)
• Finger Flexors: A2 T2 200/90/130 (neutral position is 180°)
Finochietto Test
The Finochietto Test helps to differentiate extrinsic and intrinsic hand muscle spasticity and shortening.
Box and Block Test with Video Recording
The Box and Block Test is used to test functioning.
Decision Point:
BOTOX® Muscle Selection and Dosing
You have taken a look at the results of Marie’s assessments.
BOTOX® Muscle Selection and Dosing
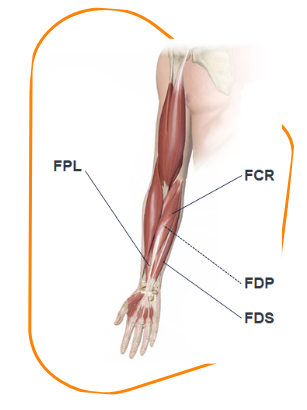
Marie received BOTOX®1 to her FPL, FDS, and FDP in doses of 20, 40, and 40 U, respectively. She did not receive any BOTOX®1 to her FCR.
See more about this decision below.
Total dose = 100 U1
FCR 0 U
(recommended dose: 15–60 U, 1–2 sites)1
FPL 20 U
(recommended dose: 20 U, 1–2 sites)1
FDS 40 U and FDP 40 U
(recommended doses: 15–50 U, 1–2 sites)1
• Ashworth 1
• Small dose to minimise the risk of finger flexor weakness interfering with prehension
FCR, flexor carpi radialis; FDP, flexor digitorum profundus; FDS, flexor digitorum sublimis; FPL, flexor pollicis longus.
Decision Point:
BOTOX® Dilution
You have seen the muscles targeted with BOTOX®1 and the doses used to treat Marie’s spasticity.
BOTOX® Dilution
The following dilution was used for Marie’s BOTOX®1 injections.
The rationale for this decision is below.
2 mL/100 U dilution
A 0.4-mL volume (20 U) was administered at each injection site.
FPL:
20 U = one injection site
FDS and FDP:
40 U = two injection sites each
FDP, flexor digitorum profundus; FDS, flexor digitorum sublimis; FPL, flexor pollicis longus.
Decision Point:
Techniques to Support BOTOX® Administration
When administering BOTOX®1, a key factor in aiming to achieve optimum outcomes is injection into the correct muscles.
Techniques to Support BOTOX® Administration
Marie’s BOTOX®1 was administered using electromyography, electrical stimulation, and ultrasound to aid accuracy, in addition, the use of all three techniques may combine the advantage of each.
The rationale for this decision is below:
Combination of Electromyography, Nerve Stimulation, and Ultrasound
Decision Point:
Adjuvant Therapies Alongside BOTOX® Treatment
You have seen the administration technique, dosage, and dilution of BOTOX®1 that Marie received.
Decision Point:
Patient Follow-up
You have decided the other treatments which may help lead to functional improvements for Marie following BOTOX®1 treatment.
Decision Point:
Assessing Treatment Effectiveness
Marie’s follow-up assessment was arranged for 2 months later.
Assessment Results
The Box and Block Test with the ABILHAND scale and GAS assessment were performed at Marie’s follow-up to determine whether she had effectively achieved her set goal.
The results of the performed assessments are given below:
• Performed with the OT to assess hand function by means of the ABILHAND scale
• Evaluates the impairment and activity level of the ICF
• At 6 weeks post-injection, Marie was able to move five more blocks in the Box and Block Test
• Obtained a score of >50
GAS, Goal Attainment Scale; ICF, International Classification of Functioning; OT, occupational therapist.
• Marie is a 35-year-old woman who has been referred to your care by her physician
• Let’s hear from Marie to find out more about why she has come to see you today
This is a hypothetical BOTOX®-treated patient case study developed in collaboration with an expert based on their experience with many patients. Case studies are intended to encourage scientific debate and exchange and to enhance appropriate patient management. Case studies are representative only, and individual patient responses may vary.
Lifestyle and Assessment
Decision Point:
Goal Setting and Patient Assessment
You have seen Marie’s clinical presentation, she is able to pick up objects but has difficulty releasing them. Based on the clinical results shown on the previous slide, a BOTOX® injection session was scheduled.1
Goal Setting
Marie has been set the following goal to achieve through her treatment.
The rationale for this decision is below.
Decision Point:
Upper Limb Assessment
You have taken a look at Marie’s current profile and the goal set to be achieved for her treatment.
Ashworth and Tardieu Scales
Finochietto Test
Box and Block Test
Jebsen-Taylor Hand Function Test
Video Recording
3D Movement Analysis
Other
*Recommended assessment measures may vary by country. Please refer to your local guidance on upper limb spasticity for further information on recommended assessments.
You have received the results of all the assessments that were performed on Marie.
Ashworth Scale and Tardieu Scale
Spasticity was assessed using the Ashworth Scale (reference international scale) and the Tardieu Scale (gives the ‘spasticity angle’ and the ‘paresis angle’, which are more sensitive to change). Spasticity was graded as follows:
• Wrist Flexors: A1 T1 130/90/120 (neutral position is 90°)
• Finger Flexors: A2 T2 200/90/130 (neutral position is 180°)
Finochietto Test
The Finochietto Test helps to differentiate extrinsic and intrinsic hand muscle spasticity and shortening.
Box and Block Test with Video Recording
The Box and Block Test is used to test functioning.
Decision Point:
BOTOX® Muscle Selection and Dosing
You have taken a look at the results of Marie’s assessments.
BOTOX® Muscle Selection and Dosing
Marie received BOTOX®1 to her FPL, FDS, and FDP in doses of 20, 40, and 40 U, respectively. She did not receive any BOTOX®1 to her FCR.
See more about this decision below.
Total dose = 100 U1
FCR 0 U
(recommended dose: 15–60 U, 1–2 sites)1
FPL 20 U
(recommended dose: 20 U, 1–2 sites)1
FDS 40 U and FDP 40 U
(recommended doses: 15–50 U, 1–2 sites)1
• Ashworth 1
• Small dose to minimise the risk of finger flexor weakness interfering with prehension
FCR, flexor carpi radialis; FDP, flexor digitorum profundus; FDS, flexor digitorum sublimis; FPL, flexor pollicis longus.
Decision Point:
BOTOX® Dilution
You have seen the muscles targeted with BOTOX®1 and the doses used to treat Marie’s spasticity.
BOTOX® Dilution
The following dilution was used for Marie’s BOTOX®1 injections.
The rationale for this decision is below.
2 mL/100 U dilution
A 0.4-mL volume (20 U) was administered at each injection site.
FPL:
20 U = one injection site
FDS and FDP:
40 U = two injection sites each
FDP, flexor digitorum profundus; FDS, flexor digitorum sublimis; FPL, flexor pollicis longus.
Decision Point:
Techniques to Support BOTOX® Administration
When administering BOTOX®1, a key factor in aiming to achieve optimum outcomes is injection into the correct muscles.
Techniques to Support BOTOX® Administration
Marie’s BOTOX®1 was administered using electromyography, electrical stimulation, and ultrasound to aid accuracy, in addition, the use of all three techniques may combine the advantage of each.
The rationale for this decision is below:
Combination of Electromyography, Nerve Stimulation, and Ultrasound
Decision Point:
Adjuvant Therapies Alongside BOTOX® Treatment
You have seen the administration technique, dosage, and dilution of BOTOX®1 that Marie received.
Decision Point:
Patient Follow-up
You have decided the other treatments which may help lead to functional improvements for Marie following BOTOX®1 treatment.
Decision Point:
Assessing Treatment Effectiveness
Marie’s follow-up assessment was arranged for 2 months later.
Assessment Results
The Box and Block Test with the ABILHAND scale and GAS assessment were performed at Marie’s follow-up to determine whether she had effectively achieved her set goal.
The results of the performed assessments are given below:
• Performed with the OT to assess hand function by means of the ABILHAND scale
• Evaluates the impairment and activity level of the ICF
• At 6 weeks post-injection, Marie was able to move five more blocks in the Box and Block Test
• Obtained a score of >50
GAS, Goal Attainment Scale; ICF, International Classification of Functioning; OT, occupational therapist.
aMildly increased muscle stiffness is a Modified Ashworth Scale (MAS) 1 or +1, while moderately is MAS 2, markedly is MAS 3, and severe is MAS 4* (see Bohannon RW, et al. 1987 for more information19).
bMeasured using the Fugl-Meyer Upper Extremity Scale3 (see Fugl-Meyer AR, et al. 1975 for more information11).
cMuscle contractions may occur due to spasms, disturbed reciprocal inhibition, or spastic dystonia and should be differentiated from contractures
dVisual inattention includes hemianopsia, scotoma, or visual neglect.
eCan be measured with the Barthel Index (low score) and EQ-5D (low score).8
The PSS Referral Tool was created with the assistance of a group of international experts in the field of PSS, utilising both published risk factors and their own clinical experience.
Publications related to the tool:
Wissel J, Allison A, Bavikatte G, et al. Development of an Early Identification Tool in Post-Stroke Spasticity (PSS): The PSS Risk Classification System. Poster presented at the DGN Congress, 25–28 September 2019, Stuttgart, Germany
References
1. AbbVie Limited. BOTOX® Summary of Product Characteristics. (Accessed: November 2022).
2. Feneis H, et al. Pocket Atlas of Human Anatomy. 4th ed. Stuttgart; Germany: Thieme Publishing Group. 2000. ISBN-13: 978-3135112046.
3. Royal College of Physicians. Spasticity in adults: management using botulinum toxin. National guidelines. 2018. Available at: https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin (Accessed: November 2022).
4. Santamato A. Int J Phys Med Rehabil Editorial. 2016;4(5).
5. Atenolol 100 mg/25 mg film-coated tablets. Summary of Product Characteristics. Gerard Laboratories. 2018. (Accessed: December 2022).
6. Metformin 1000 mg film-coated tablets. Summary of Product Characteristics. Merck Ltd. 2022. (Accessed: December 2022).
7. Catapano AL, et al. Eur Heart J. 2016;37(39):2999–3058.
8. Wissel J, Verrier M, Simpson DM, et al. Post-stroke spasticity: Predictors of early development and considerations for therapeutic intervention. PM R. 2015;7:60–67.
9. Sunnerhagen KS. Predictors of spasticity after stroke. Curr Phys Med Rehabil Rep. 2016;4:182–185.
10. Opheim A, Danielsson A, Murphy MA, et al. Early prediction of long-term upper limb spasticity after stroke. Neurology. 2015;85:873–880.
11. Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. Scand J Med. 1975;7:13–31.
12. Wilkinson D, Sakel M, Camp SJ, et al. Patients with hemispatial neglect are more prone to limb spasticity, but this does not prolong their hospital stay. Arch Phys Med Rehabil. 2012;93:1191–1195.
13. De Jong LD, Hoonhorst MH, Stuive I, et al. Arm motor control as predictor for hypertonia after stroke: A prospective cohort study. Arch Phys Med Rehabil. 2011;92:1411–1417.
14. Nijland RH, va Wegen EE, Harmeling-van der Wel BC, et al. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study Stroke. 2010;41:745–750.
15. Wissel J, Ward AB, Erztgaard P, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med. 2009;41:13–25.
16. NICE guideline: Stroke rehabilitation in adults. June 2013. Available at: https://www.nice.org.uk/guidance/cg162/resources/stroke-rehabilitation-in-adults-pdf-35109688408261 (Accessed: December 2022).
17. Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:e100–43.
18. Leathley MJ, Gregson JM, Moore AP, et al. Predicting spasticity after stroke in those surviving to 12 months. Clin Rehabil. 2004;18:438–443.
19. Bohannon RW and Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207.
20. Royal College of Physicians. National clinical guideline for stroke. 2016. Available at: https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx (Accessed: December 2022).