BOTOX® has safety and tolerability experience from +30 years of use in a range of conditions, with 10 years in chronic migraine5-7
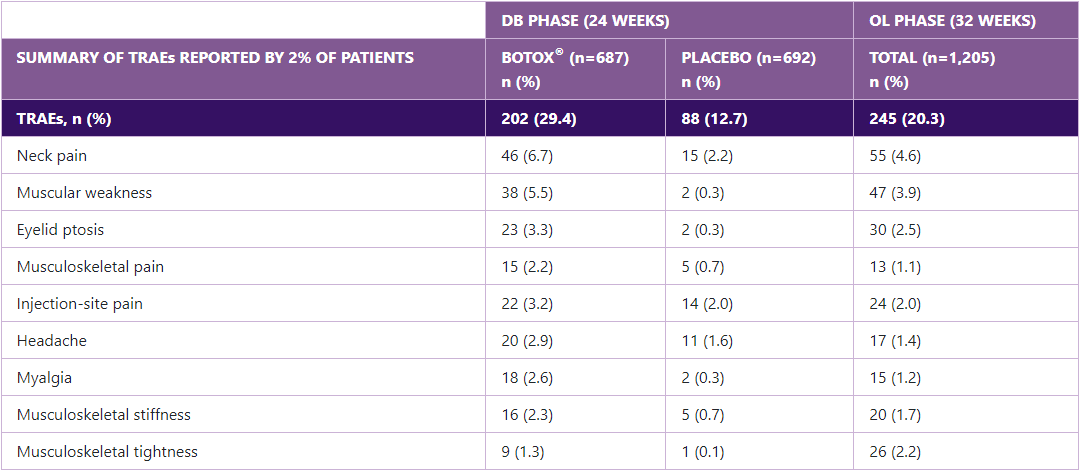
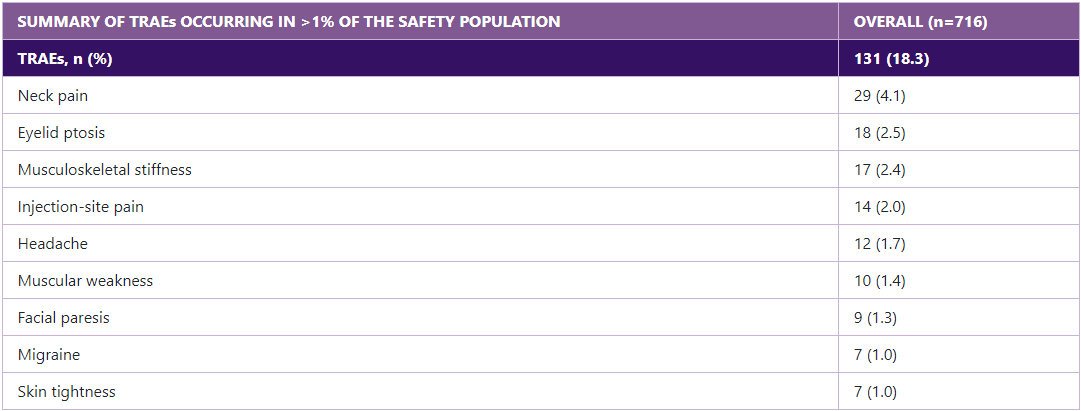
Adverse events in the PREEMPT and COMPEL studies
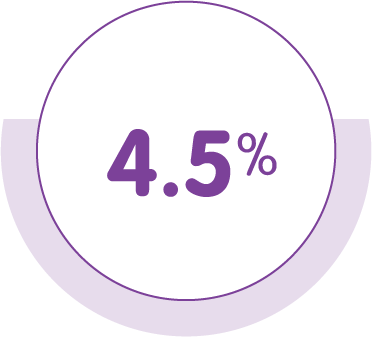
Discontinuation rates due to adverse events in the PREEMPT and COMPEL studies
The rate of treatment-emergent adverse events progressively decreases with subsequent rounds of BOTOX® treatment.9
DB: double blind; CM: chronic migraine; OL: open label; TRAE: treatment-related adverse event.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: March 2024. UK-BCM-240040.