This promotional material is intended for UK Healthcare Professionals only. BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
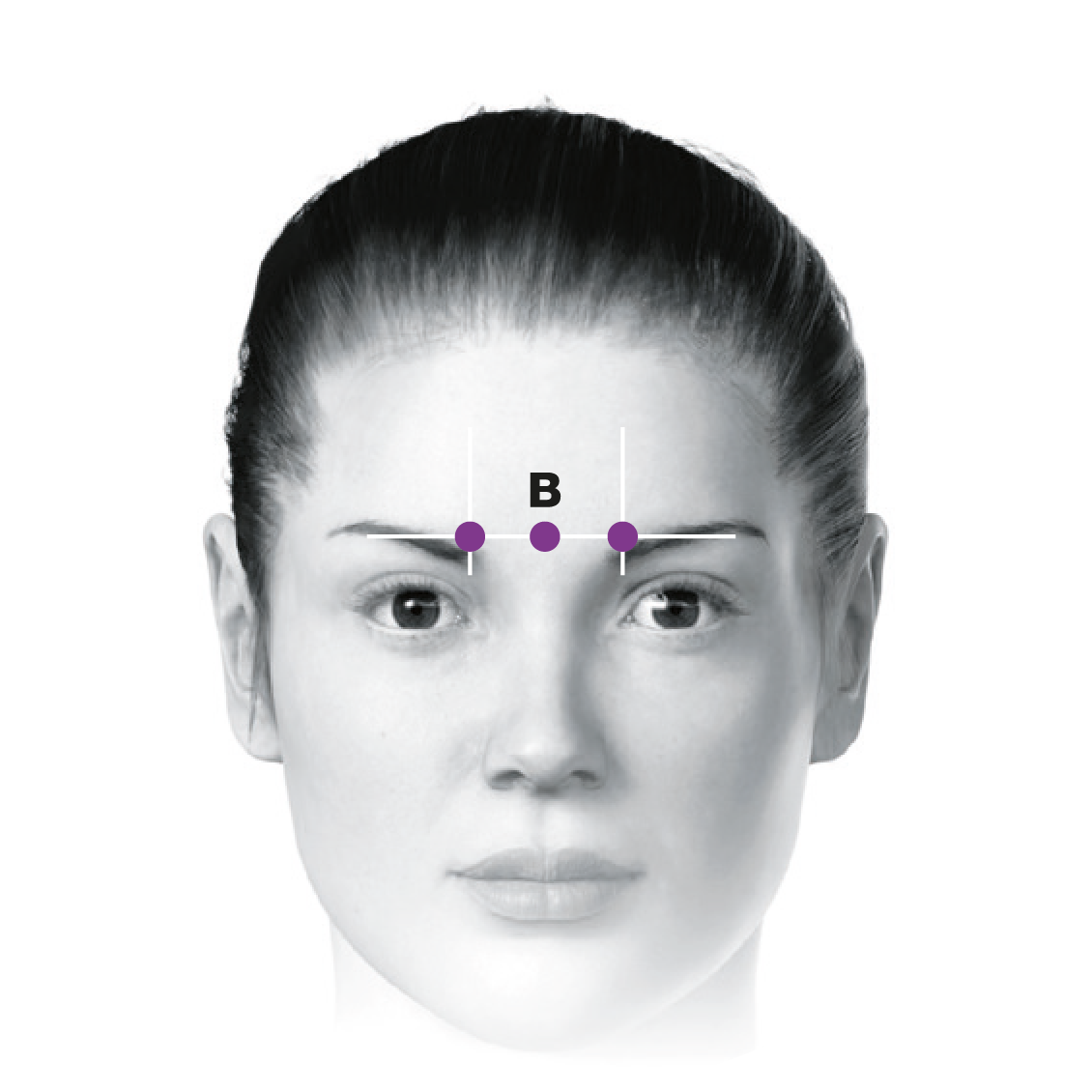
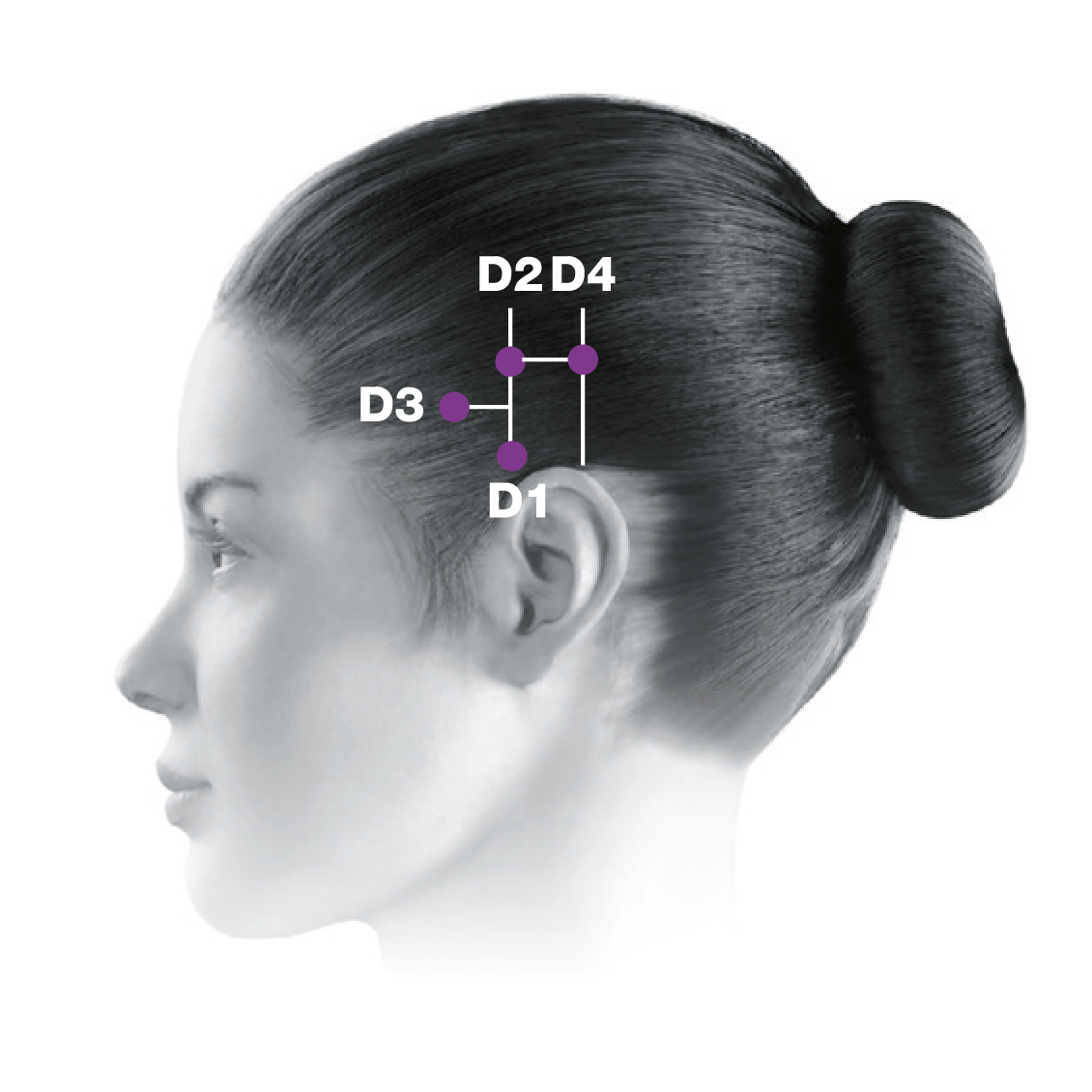
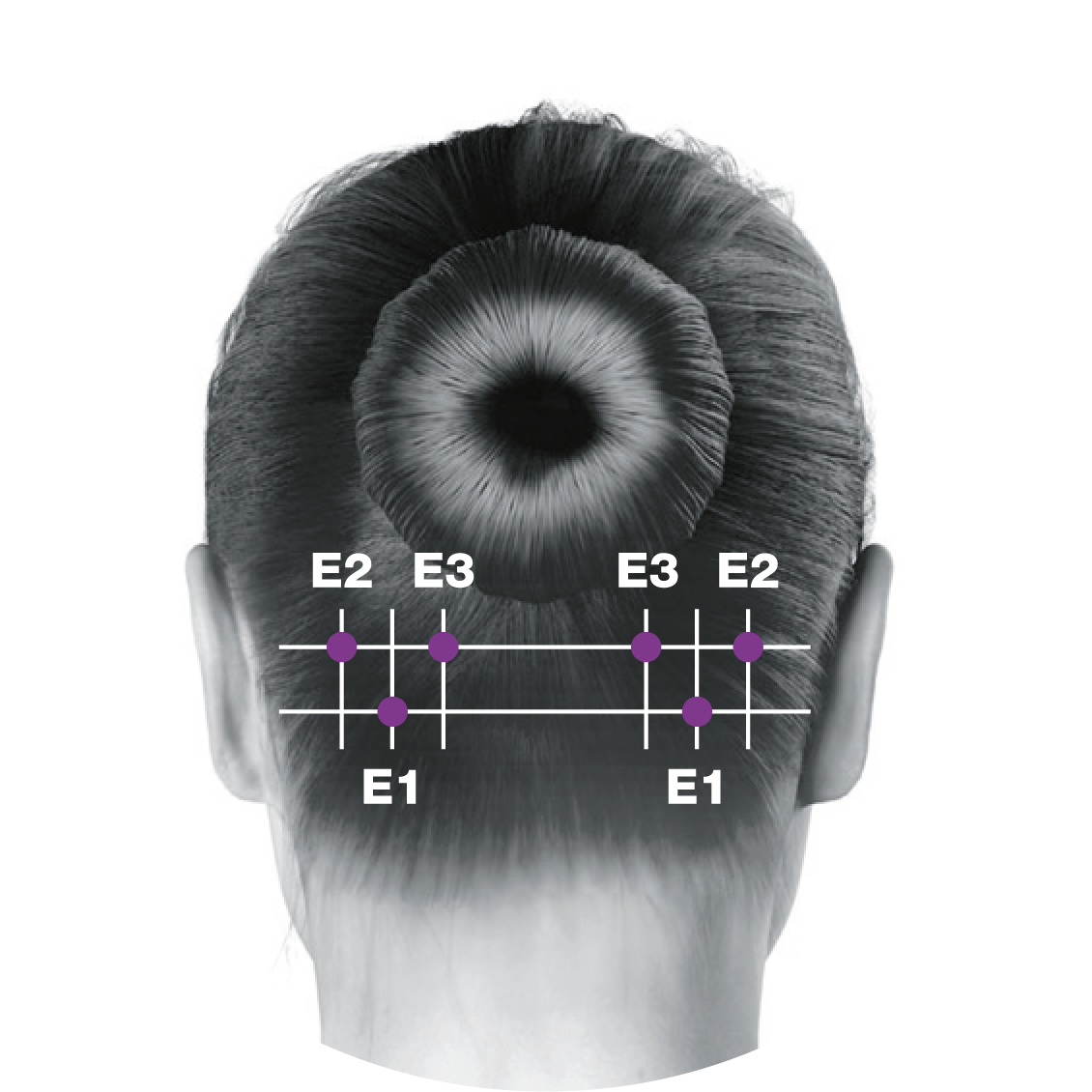
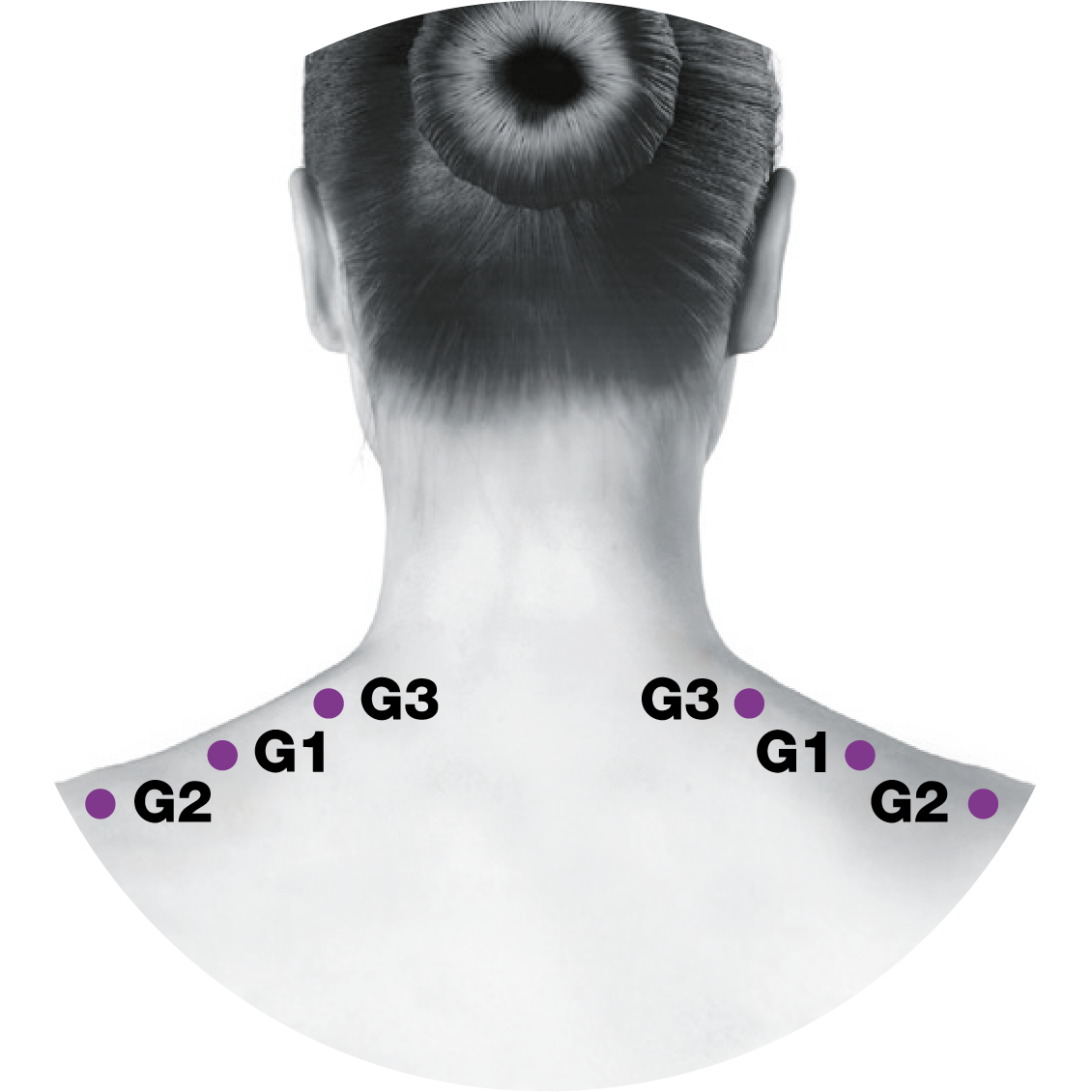
Administering BOTOX® to your patients with chronic migraine5-7
This treatment paradigm is based on the PREEMPT study protocol, which is the culmination of 10 years’ experience in migraine research2,5-13
*Follow the pain.
0.1 mL (5 Units) of BOTOX® per site. Please refer to the dilution table in your Summary of Product Characteristics (SmPC). The recommended dose for treating chronic migraine is 155 U to 195 U. BOTOX® should be individualised for each patient.5
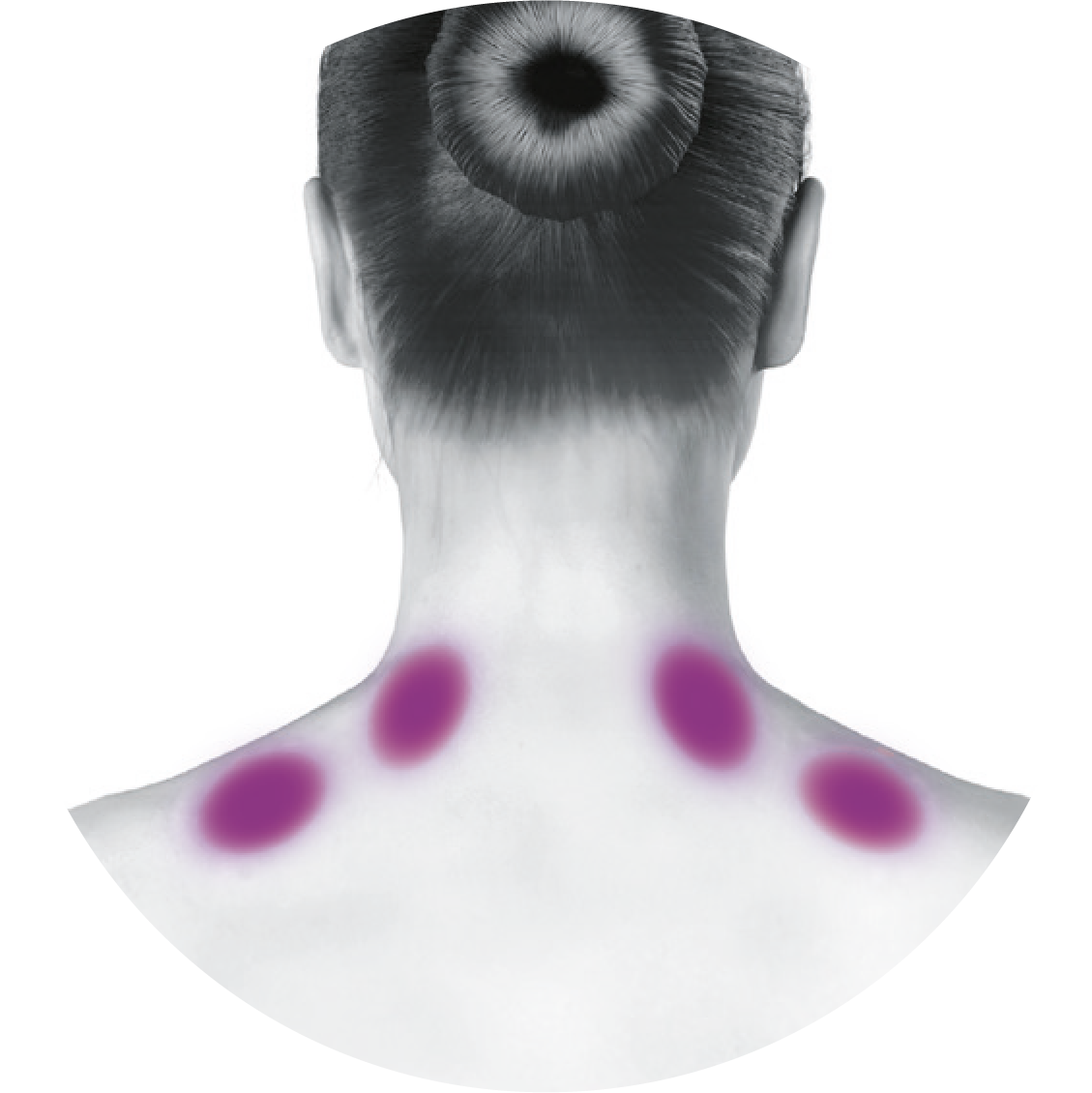
Based on a publication by Blumenfeld A M, et al. Headache 2017;57(5):766-777,7 the injection points demonstrated in the images differ from the images shown in the BOTOX® product licence. Importantly, these are not changes to the muscle groups identified in the licence, but simply reflect an amendment in technique to localise these muscles groups more effectively and minimise side effect profile.
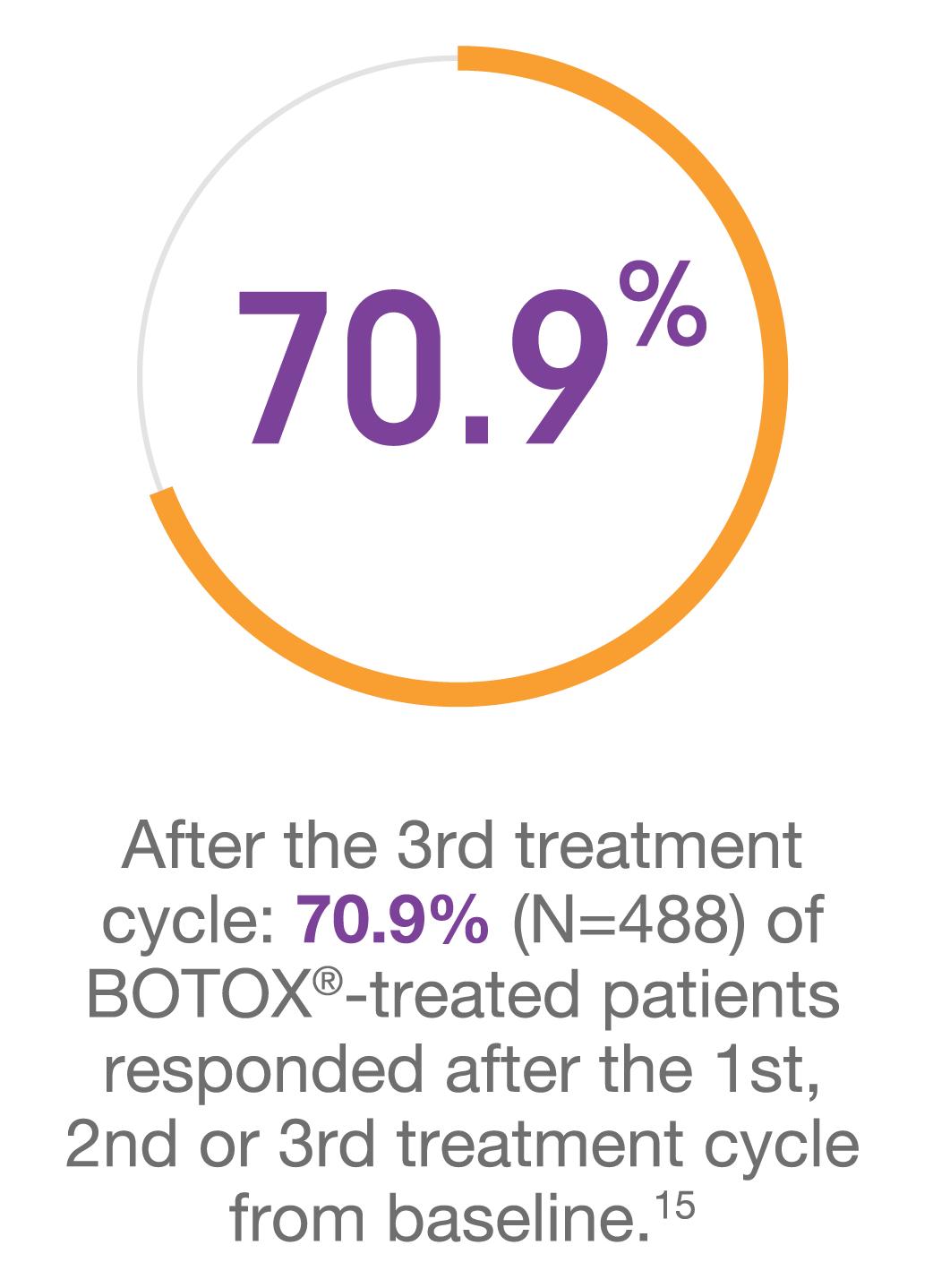
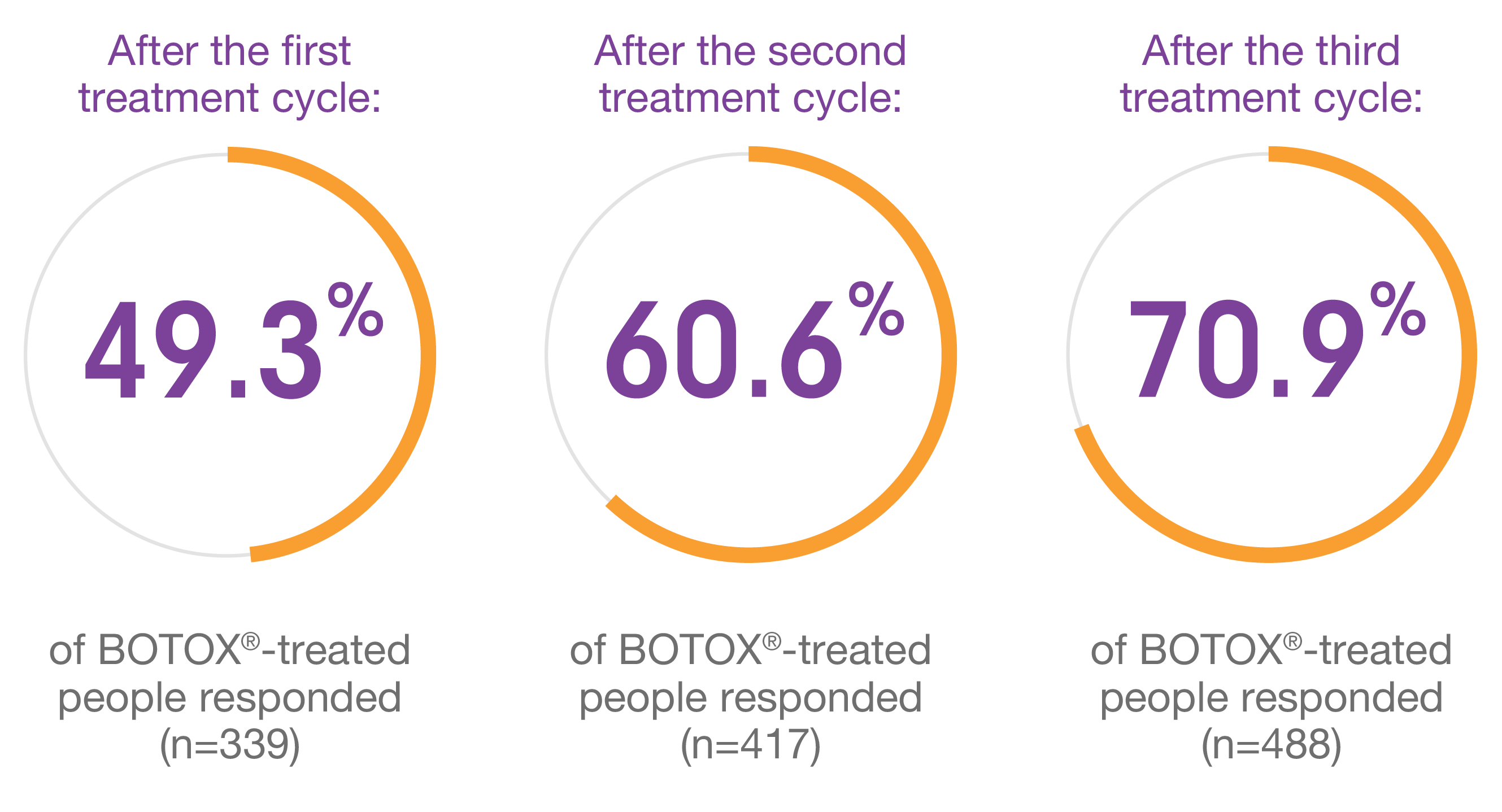
The nature of chronic migraine and progressive response with BOTOX® makes treatment persistence important14,15
CM: chronic migraine.
†Response was defined as a ≥50% reduction in monthly headache days from baseline.15
‡Primary endpoint was mean change from baseline in frequency of headache days.15
§Cumulative response of first time responders. First-time responders for a given time point are patients who never responded at any previous time points.15
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: August 2025. UK-BCM-240120.