This promotional material is intended for UK Healthcare Professionals only. BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
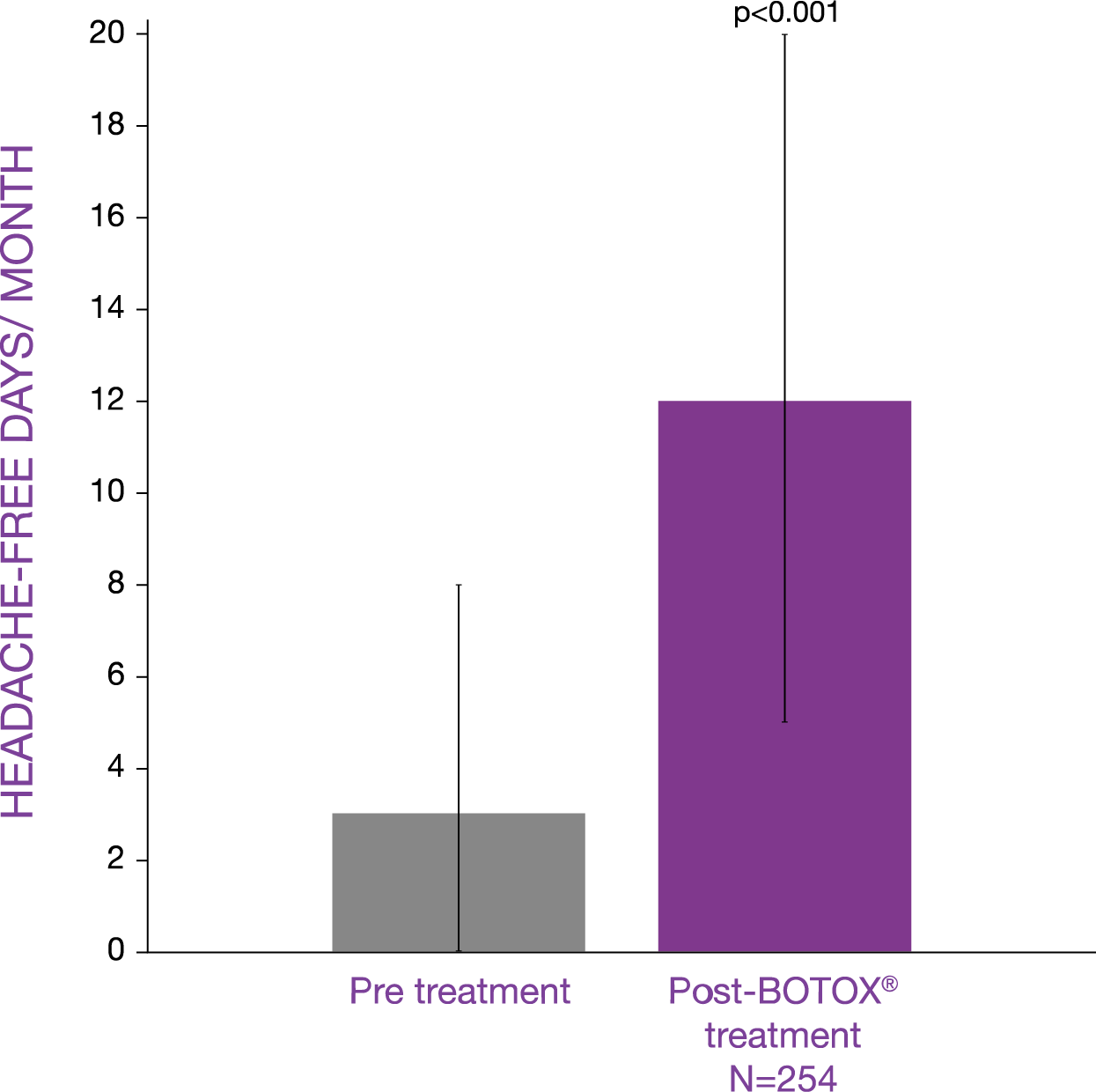
BOTOX® delivers a clinically meaningful reduction in headache frequency for those with chronic migraine…6
…and with four times more headache-free days after BOTOX® initiation, patients can focus on enjoying their lives.6
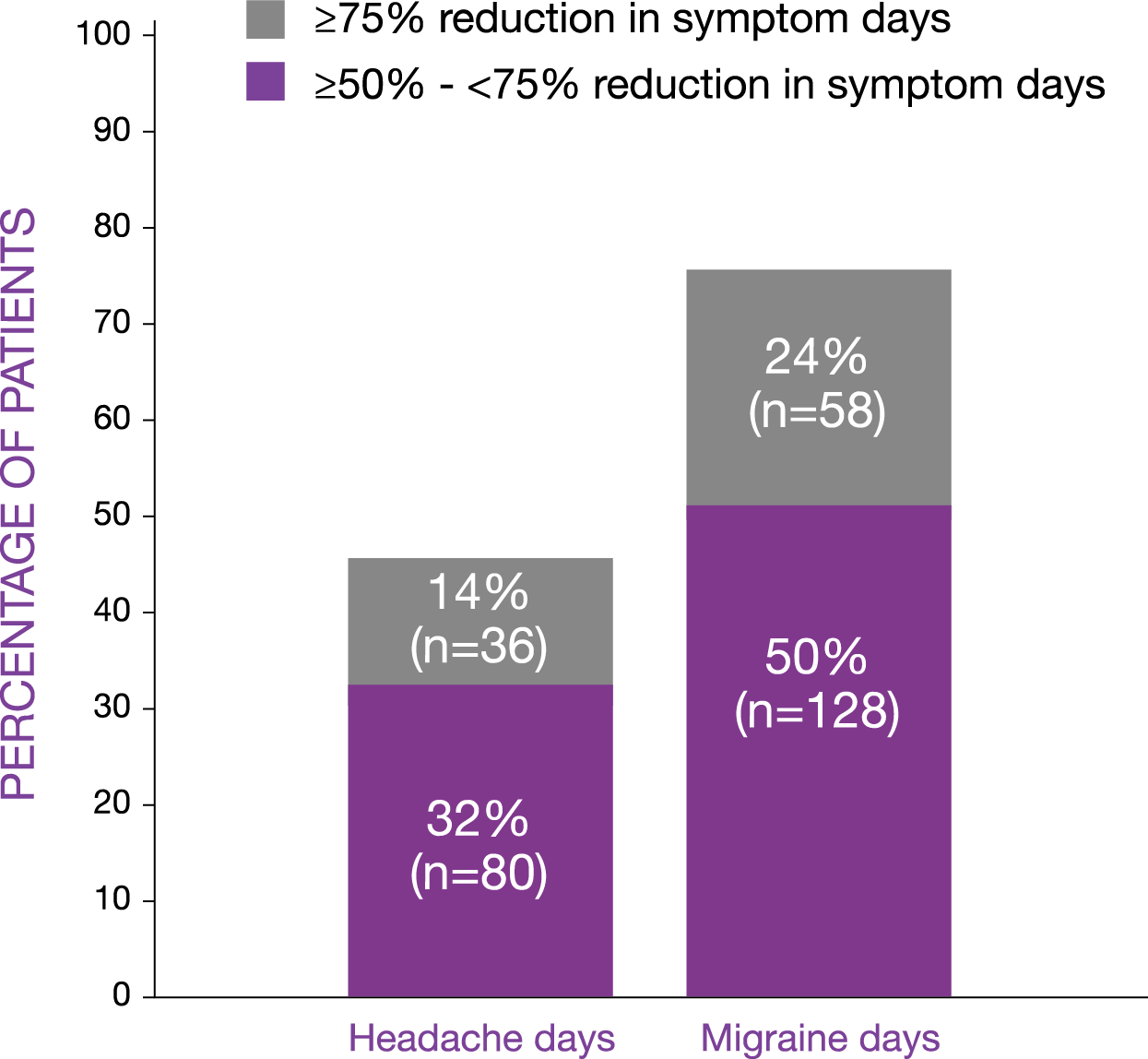
In a UK, prospective, real-world study, BOTOX®:6
- Reduced median headache days per month from 27 to 18 and median migraine days per month from 15 to 7, baseline versus after treatment (p<0.001)
- Significantly increased the number of monthly headache-free days, compared with pre-treatment, in people with chronic migraine (p<0.001)
Study design
254 adults in the UK with chronic migraine received BOTOX® (botulinum toxin type A) between July 2010 and May 2013, and their headache data were collected and analysed. The investigators examined headache, migraine days decrements, crystal clear days increment in the month post treatment and 50% responder rate.6
BOTOX®: Proven efficacy for patients with chronic migraine in the real world and in clinical trials3,4,7,8
- In a 6-month, placebo-controlled trial, BOTOX® significantly reduced headache days from baseline compared with placebo (-8.4 vs -6.6, p<0.001; N=1,384; primary endpoint for the pooled analysis)3
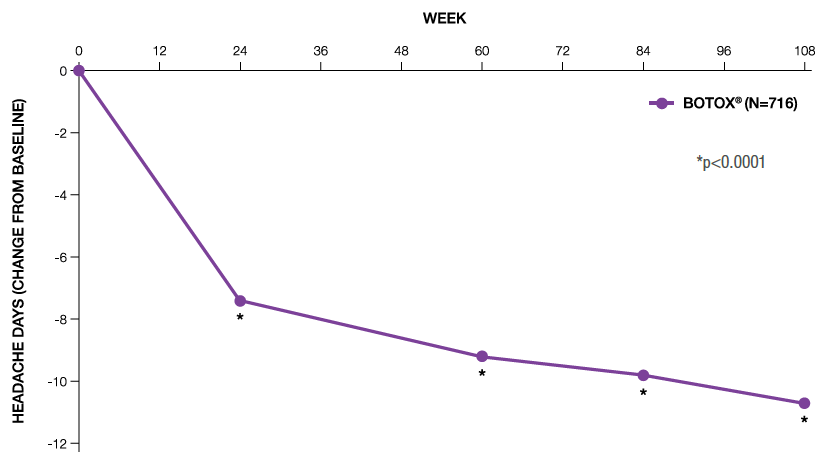
- In a 2-year open-label study, BOTOX® progressively reduced headache days from baseline up to 2 years (-10.7 days, p<0.0001; N=716). Patients reported an average of 22 headache days per month at baseline4
BOTOX® significantly reduces headache days up to 108 weeks4
Adapted from Blumenfeld A M et al, 20184
Study details:
COMPEL is an international, multicentre, open-label long-term prospective study. Adult patients with chronic migraine received BOTOX® (155 U) every 12 weeks for nine treatment cycles. The primary endpoint was headache day reductions at 108 weeks.4
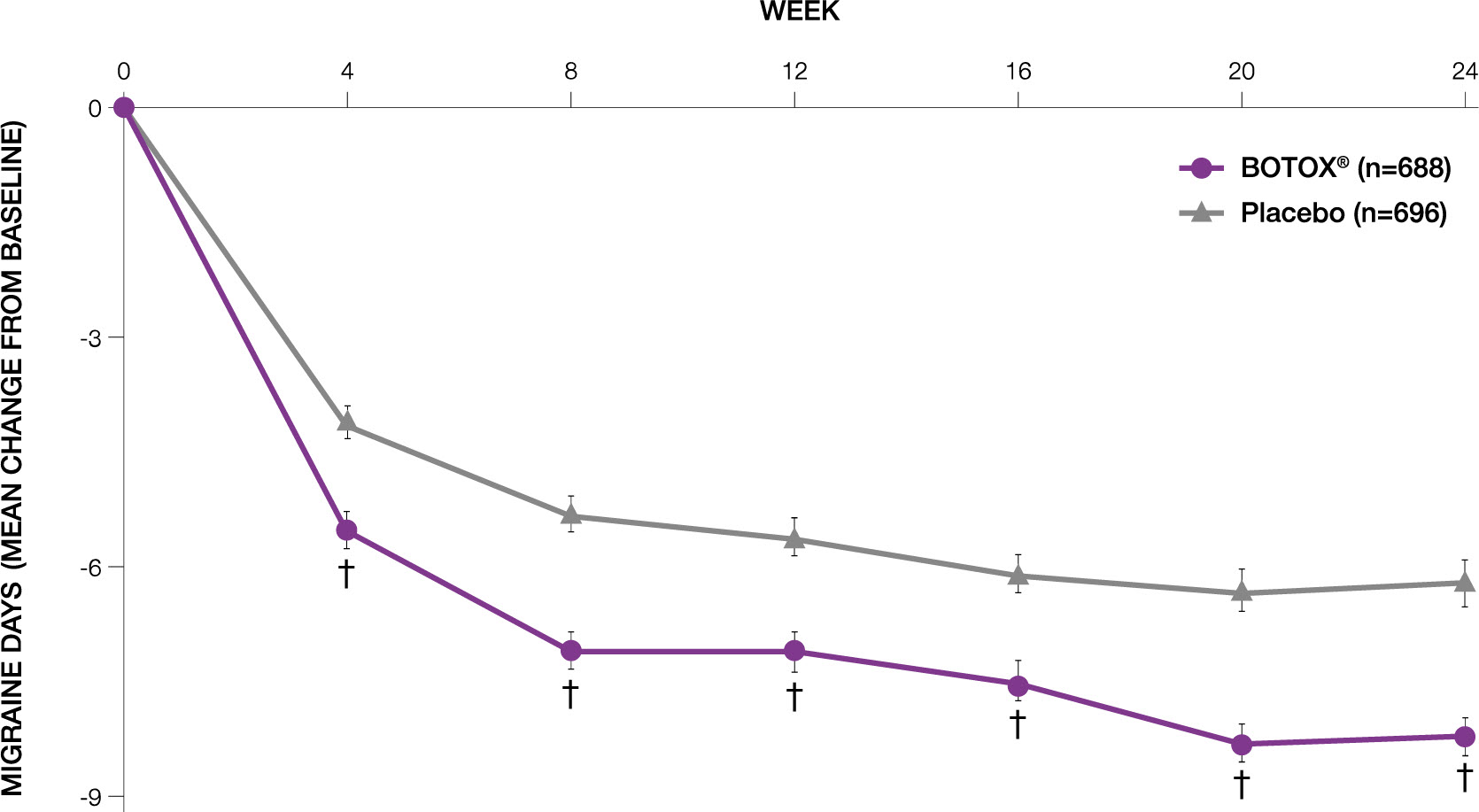
- BOTOX® significantly reduces the frequency of migraine days compared with placebo up to 24 weeks (p<0.001; N=1,384)7
BOTOX® significantly reduces migraine days compared with placebo7
Adapted from Dodick D W et al, 20107
†p<0.001.
Includes migraines with and without aura and probable migraines.7
Study details:
Pooled analysis of the two multicentre pivotal trials (PREEMPT), with a 24-week randomised, double-blind phase followed by a 32-week open-label phase. Patients were randomised (1:1) to BOTOX® (155-195 U) or placebo injections every 12 weeks. The primary endpoint for the pooled analysis was mean change from baseline in frequency of headache days for the 28-day period ending with week 24.7
In PREEMPT at baseline the mean frequency of migraine days was 19.1 in the BOTOX® arm and 18.9 in the placebo arm.7
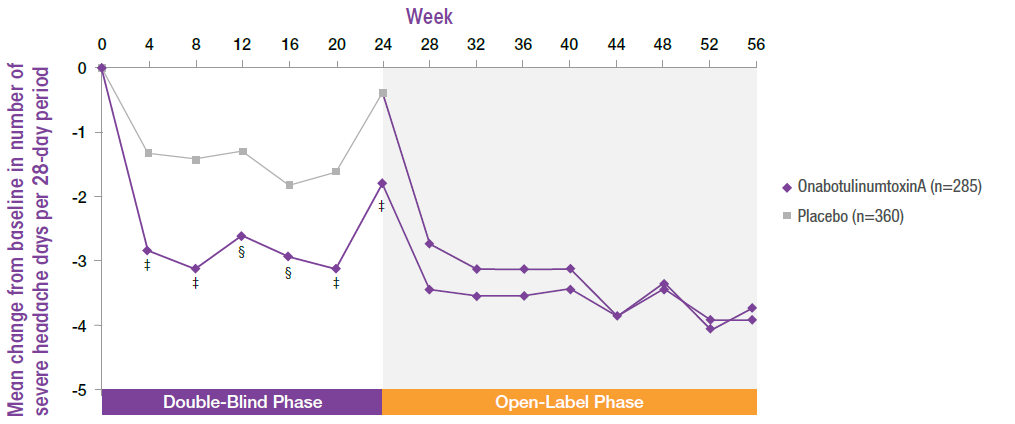
- BOTOX® significantly reduced the number of severe headache days experienced up to Week 24 compared with placebo8
- The number of severe headache days continued to decline during the open-label phase up to Week 568
BOTOX® significantly reduces severe headache days compared with placebo in a sub-group of patients who were non-responders for headache days
Adapted from Matharu M et al, 20178
‡p<0.001 versus placebo. §p<0.05 versus placebo.
Non-responders were defined as those that achieved <50% reduction in headache days.
Study details:
56-week pooled analysis of the PREEMPT trials, including a 24-week double-blind phase and the 32-week open-label phase. Patients with chronic migraine were randomised 1:1 to BOTOX® (155-195 U) or placebo injections every 12 weeks.8
CM: chronic migraine.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BCM-250069.