This promotional material is intended for UK Healthcare Professionals only. BOTOX® (botulinum toxin type A) Prescribing Information and adverse event reporting information can be found below.
BOTOX®: prophylaxis treatment option for the management of chronic migraine6-26
BOTOX® could have a positive impact on the lives and future outlook of your patients with
chronic migraine4,6,7
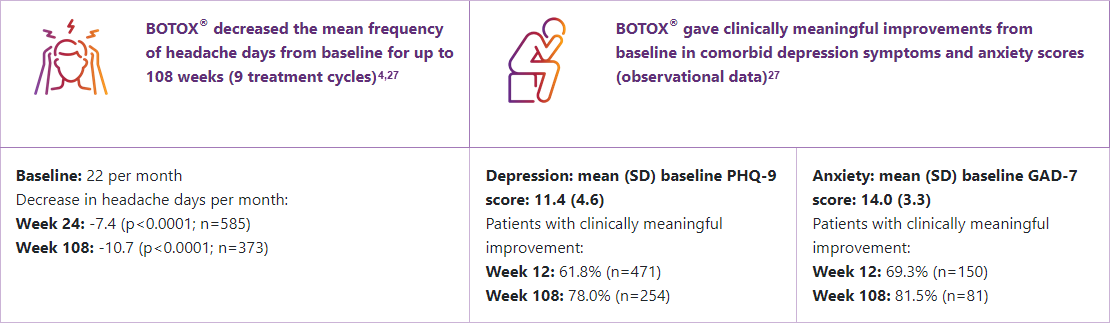
In an international, multicentre, long-term, open-label, single-arm prospective study in patients with chronic migraine:4, 27
CM: chronic migraine; FDA: Food and Drug Administration; GAD-7: 7-item Generalized Anxiety Disorder Assessment; IQR: interquartile range; PHQ-9: Patient Health Questionnaire-9; SD: standard deviation.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use. The BOTOX® Summary of Product Characteristics can be found here
By clicking the link above you will leave the AbbVie Pro website and be taken to the eMC PI portal website.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: June 2025. UK-BCM-250065.