Safety Profile in Psoriasis
Learn more about the safety profile of SKYRIZI in clinical trials and long-term studies for psoriasis and psoriatic arthritis.
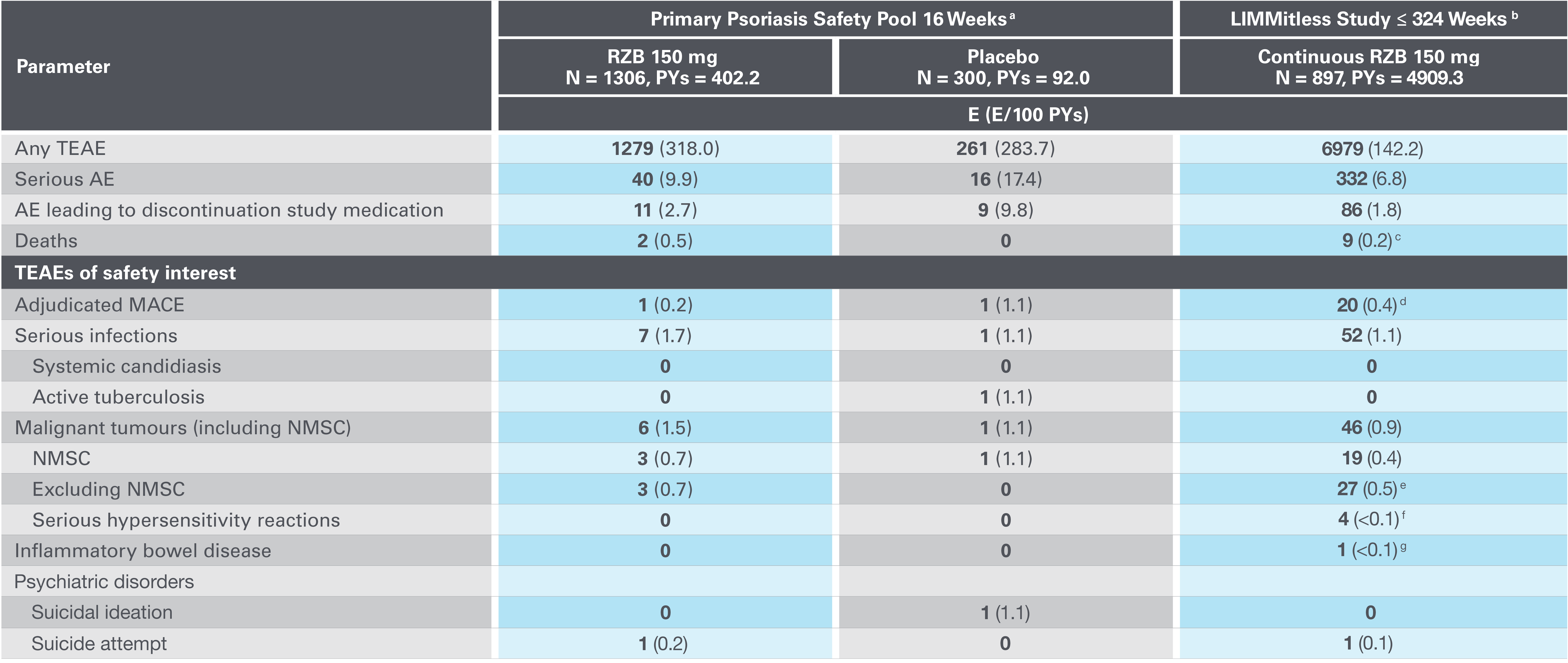
*Contraindications include hypersensitivity to the active substance or to any of the excipients and clinically important active infections e.g. active tuberculosis.
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Featured content
UK-RISN-240171. Date of preparation May 2024.
References
- Reich K, et al. Lancet 2019; 394: 576-586.
- Gordon KB, et al. Lancet 2018; 392: 650-661.
- Warren RB, et al. Risankizumab vs Secukinumab in Patients with Moderate-to-Severe Plaque Psoriasis: A Phase 3 Trial, Presented at AAD 2020.
- SKYRIZI: Summary of Product Characteristics.
- Strober B, et al. Poster 1714, presented at the 28th European Academy of Dermatology and Venereology (EADV) Congress, 9-13 October 2019, Madrid, Spain
- Reich K, et al. Poster 1813, Efficacy and Safety of Risankizumab Compared with Adalimumab in Patients with Moderate-to-Severe Plaque Psoriasis: Results from the Phase 3 IMMvent Trial. 2018.
- Papp KA, et al. Long-Term Safety and Efficacy of Risankizumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: Interim Analysis of the LIMMitless Open-label Extension Trial for up to 6 Years of Follow-up. Presented at EADV, 11–14 October 2023, Berlin, Germany; P2428.
- Kristensen LE, et al. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 52-Week Results From the KEEPsAKE 1 and KEEPsAKE 2 Trials. Oral presentation EADV 30th Congress 2021 - Anniversary Edition 29 Sept – 2 Oct 2021.
UK-RISN-240160. Date of preparation: May 2024.