Find out more about the efficacy of SKYRIZI
Explore the durability of response with SKYRIZI through clinical trials and long-term data in psoriasis and psoriatic arthritis. Start by selecting a study from the menu below.
LONG-TERM DATA
HEAD-TO-HEAD TRIALS
SKYRIZI IN PsA
SKYRIZI LONG-TERM DATA: LIMMitless
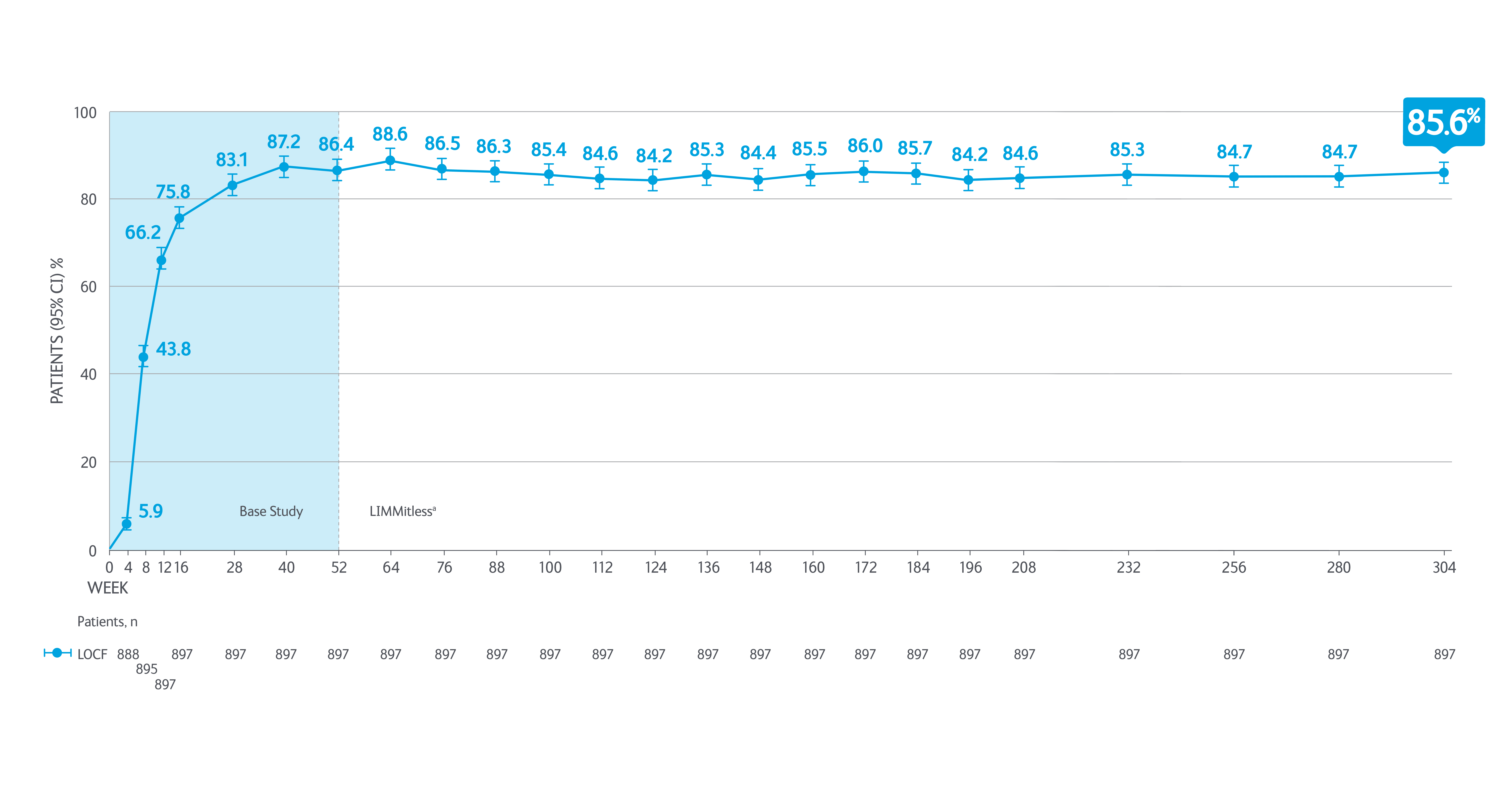
Durable efficacy in psoriasis achieved by SKYRIZI patients through to 5.8 years*5
>85% of SKYRIZI patients achieved PASI 90 at 304 weeks*5
Adapted from Papp K, et al. 2023.
aBecause of differences in base study lengths, some patients enrolled in the LIMMitless study earlier than 52 weeks.
*Results from 304 week interim analysis including integrated data from five Phase II/III studies (UltIMMA-1, UltIMMa-2, SustaIMM, IMMvent, and NCT03255382) and the LIMMitless study.
Efficacy and safety assessments were performed every 12 weeks until week 156 and every 24 weeks thereafter. Efficacy was assessed by PASI 90, PASI 100, sPGA 0/1, mean PASI percent improvement. Quality of life was assessed by DLQI 0/1. LOCF (last observation carried forward): used completed evaluation from the most recent visit to impute missing data at later visits. PASI 90 = 90% improvement in Psoriasis Area and Severity Index.
DLQI, Dermatology Life Quality Index; LOCF, last observation carried forward; PASI, Psoriasis Area Severity Index; sPGA, static Physicians Global Assessment.
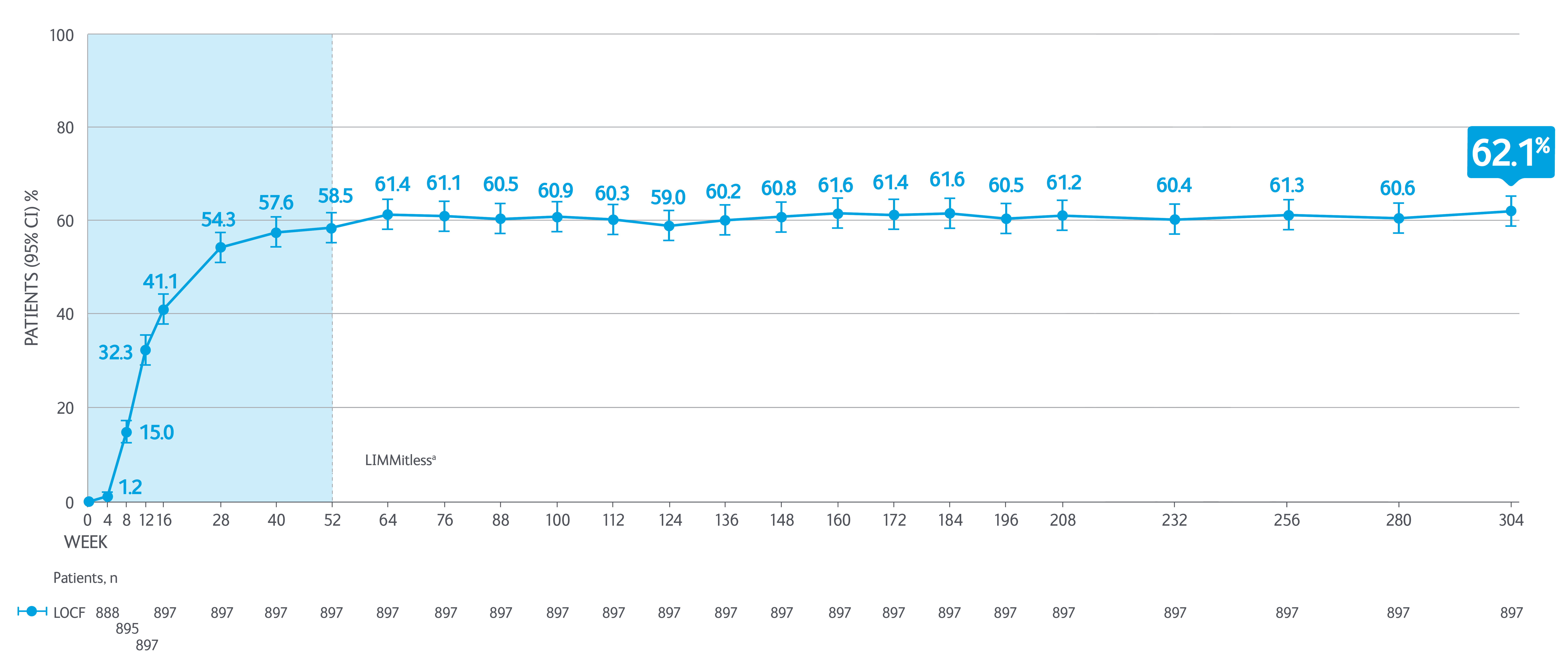
>60% of SKYRIZI patients achieved PASI 100 at 304 weeks*5
Adapted from Papp K, et al. 2023.
aBecause of differences in base study lengths, some patients enrolled in the LIMMitless study earlier than 52 weeks.
*Results from 304 week interim analysis including integrated data from five Phase II/III studies (UltIMMA-1, UltIMMa-2, SustaIMM, IMMvent, and NCT03255382) and the LIMMitless study.
Efficacy and safety assessments were performed every 12 weeks until week 156 and every 24 weeks thereafter. Efficacy was assessed by PASI 90, PASI 100, sPGA 0/1, mean PASI percent improvement. Quality of life was assessed by DLQI 0/1. LOCF (last observation carried forward): used completed evaluation from the most recent visit to impute missing data at later visits. PASI 100 = 100% improvement in Psoriasis Area and Severity Index.
DLQI, Dermatology Life Quality Index; LOCF, last observation carried forward; PASI, Psoriasis Area Severity Index; sPGA, static Physicians Global Assessment.
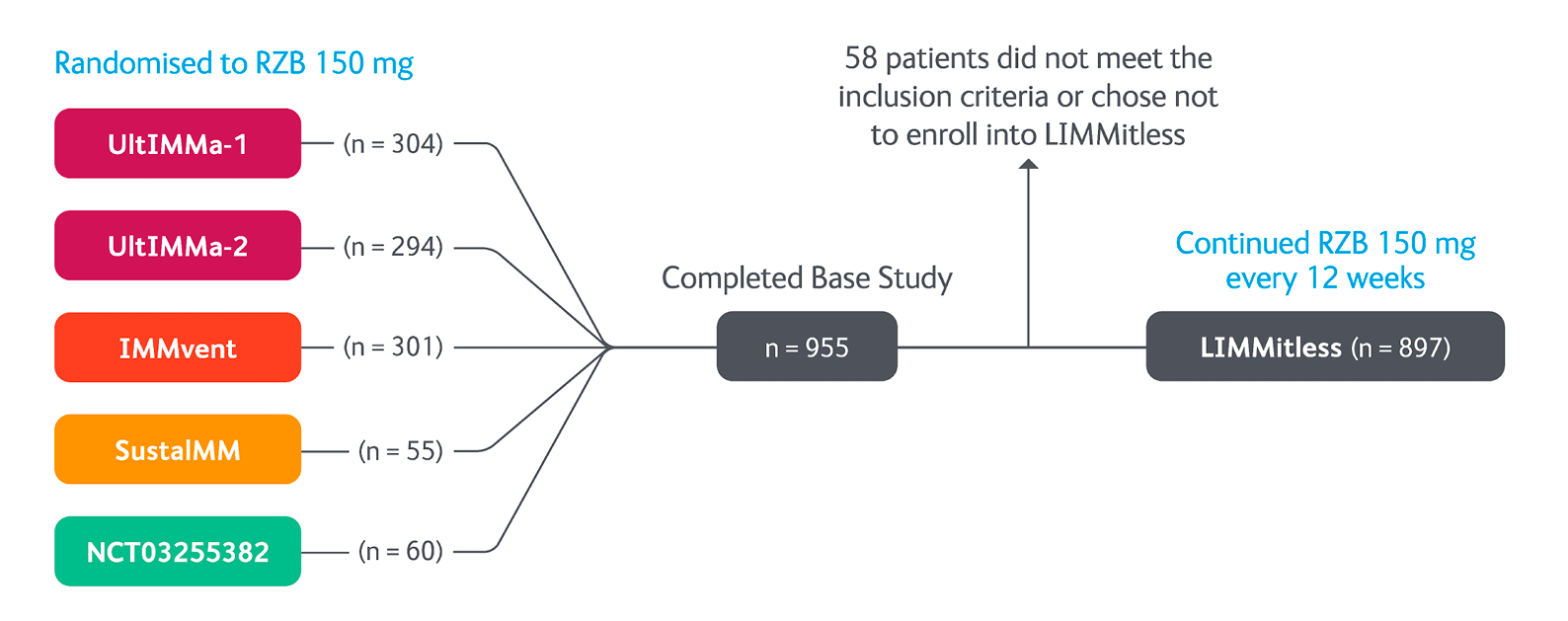
LIMMitless OLE Study Design5
LIMMitless is an ongoing Phase III, single-arm, multicentre, international, open-label extension in which all patients received SKYRIZI (150 mg) every 12 weeks. The 304 week analysis included patients who were initially randomised to receive SKYRIZI (150 mg) in 1 of 5 base Phase II/III studies. Results from 304 week interim analysis included integrated data from five Phase II/III studies (UltIMMa-1, UltIMMa-2, SustaIMM, IMMvent, and NCT03255382) and the LIMMitless study. Efficacy and safety assessments were performed every 12 weeks until week 156 and every 24 weeks thereafter. Efficacy was assessed by PASI 90, PASI 100, sPGA 0/1, mean PASI percent improvement. Quality of life was assessed by DLQI 0/1.
Adapted from Papp K, et al. 2023.
DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area Severity Index; RZB, risankizumab; sPGA, static Physicians Global Assessment.
Efficacy and safety assessments were performed every 12 weeks until week 156 and every 24 weeks thereafter:
− PASI 90
− PASI 100
− sPGA 0/1
− Mean PASI percent improvement
− DLQI 0/1
Safety was assessed via adverse event monitoring through the cutoff date for this analysis (May 22, 2023)
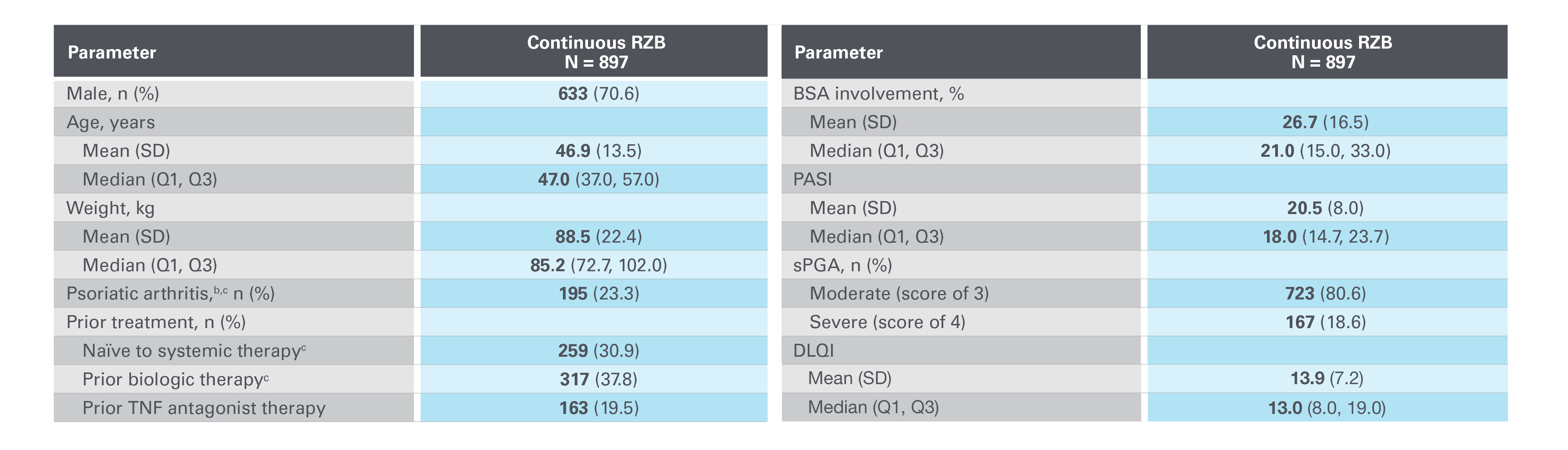
LIMMitless OLE baseline demographicsa5
Adapted from Papp K, et al. 2023.
BSA, body surface area; DLQI, Dermatology Life Quality Index; OLE, open-label extension; PASI, Psoriasis Area and Severity Index; Q1/Q3, quartile 1/3; RZB, risankizumab; SD, standard deviation; sPGA, static Physician’s Global Assessment; TNF, tumour necrosis factor.
aBaseline at the start of base study.
bDiagnosed or suspected.
cBased on n=838; data not collected in NCT03255382.
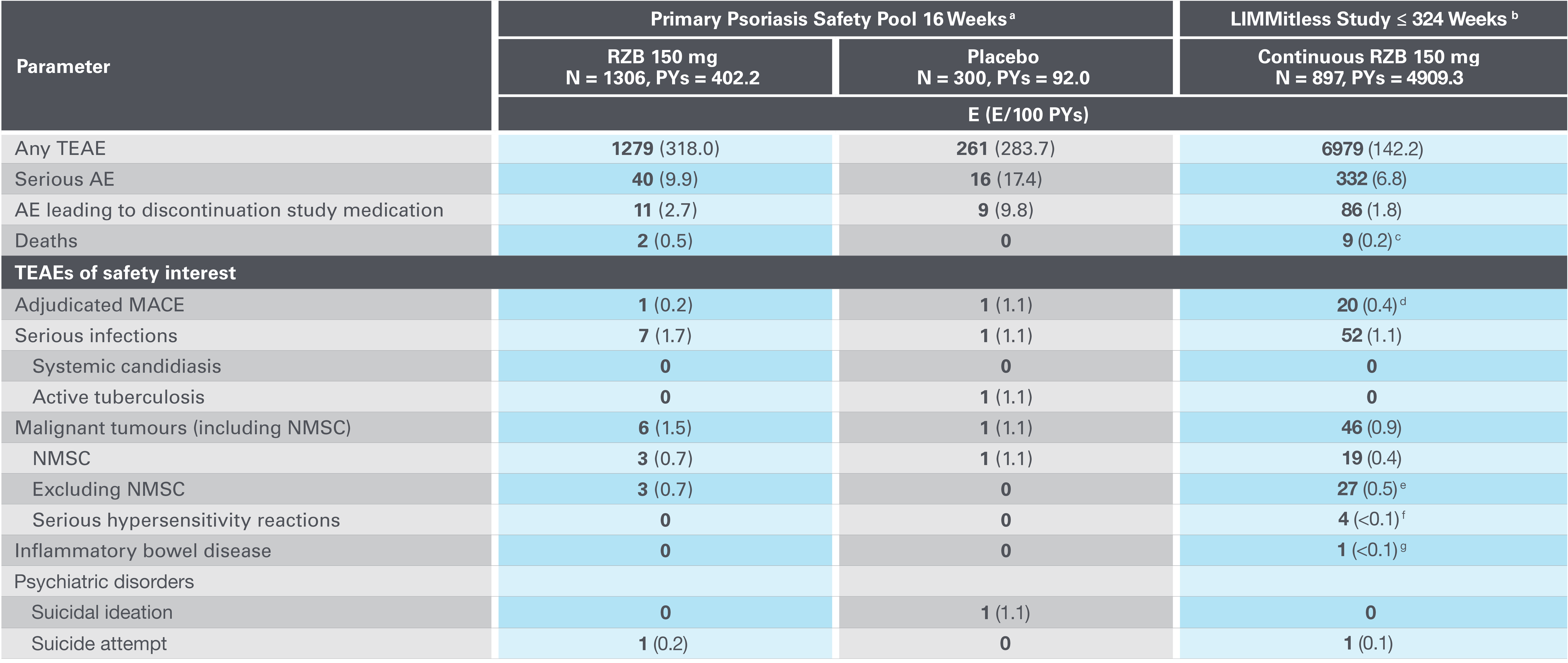
Consistent safety profile through 324 weeks5
Adapted from Papp K, et al. 2023.
AE, adverse event; E, events; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PYs, patient-years; RZB, risankizumab; TEAE, treatment-emergent AE.
aPrimary psoriasis safety pool includes UltIMMa-1, UltMMa-2, IMMhance, IMMvent and NCT0205448110 studies.
b16-week safety data for patients who received RZB 150 mg UltIMMa-1, UltMMa-2, and IMMvent (primary psoriasis safety pool) are included in the 324-week LIMMitless results.
cDue to natural causes (n=1), accident (n=1), cardiovascular event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=2), and COVID-19 infection (n=1); none related to RZB.
dMACE rate in the LIMMitless study is consistent with the incidence rate of MACE in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65).
eMalignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1).
fSerious hypersensitivity reactions (all of which were considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), and Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]).
gOne nonserious event of ulcerative colitis, considered unrelated to RZB.
Featured content
UK-RISN-250219. Date of preparation July 2025.
References
- Reich K, et al. Lancet 2019; 394: 576-586.
- Gordon KB, et al. Lancet 2018; 392: 650-661.
- Warren RB, et al. Risankizumab vs Secukinumab in Patients with Moderate-to-Severe Plaque Psoriasis: A Phase 3 Trial, Presented at AAD 2020.
- SKYRIZI: Summary of Product Characteristics.
- Papp KA, et al. Long-Term Safety and Efficacy of Risankizumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: Interim Analysis of the LIMMitless Open-label Extension Trial for up to 6 Years of Follow-up. Presented at EADV, 11–14 October 2023, Berlin, Germany; P2428.
UK-RISN-240140. Date of preparation: May 2024.