▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Vyalev™ (foslevodopa and foscarbidopa solution for infusion) Indication and Summary of Important Treatment Considerations
Indication
Treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.
Contraindications
VYALEV™ is contraindicated in patients with hypersensitivity to the active substances or to any of the excipients, narrow‑angle glaucoma, severe heart failure, acute stroke, severe cardiac arrhythmia, comedication with selective MAO type A inhibitors and nonselective MAO inhibitors, conditions contraindicated for adrenergics (e.g. pheochromocytoma, hyperthyroidism, and Cushing’s syndrome), and suspicious undiagnosed skin lesions or history of melanoma.
Select special warnings and precautions for Vyalev™
Special warnings and precautions for Vyalev™
Several warnings and precautions below are generic for levodopa and, therefore, also for Vyalev™
Not recommended for the treatment of drug-induced extrapyramidal reactions.
Caution use in patients with: severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions. History of myocardial infarction with residual atrial nodal or ventricular arrhythmias, cardiac function should be monitored during the initial dosage adjustments. Monitor all patients for the development of mental changes, depression with suicidal tendencies, and other serious mental changes. Caution with past or current psychosis and antipsychotics used concomitantly with dopamine receptor-blocking properties (observe for loss of antiparkinsonian effect). Higher frequency of hallucinations may occur with dopamine agonists and/or other dopaminergic treatments including Vyalev™. Monitor patients regularly for the development of impulse control disorders, for example Dopamine Dysregulation Syndrome (DDS). Before initiation of treatment, warn patients and caregivers of the potential risk of developing DDS. The dose of Vyalev™ may need to be adjusted downwards in order to avoid levodopa induced dyskinesias. Caution in chronic wide-angle glaucoma; monitor for intra-ocular pressure changes. Vyalev™ may induce orthostatic hypotension and should be given cautiously in patients taking other medicinal products that may cause orthostatic hypotension. Concomitant use of selegiline and levodopa/carbidopa has been associated with serious orthostatic hypotension. Levodopa may induce somnolence and sudden sleep: caution should be exercised when driving and operating machines. Risk of symptoms resembling Neuroleptic Malignant Syndrome following abrupt dose reduction or discontinuation.
Infusion site events (see section 4.8) have been reported in patients receiving Vyalev™. Follow aseptic techniques and frequently rotate the infusion site to reduce the risk. In clinical studies, few patients who reported infusion site reactions also experienced infusion site infections. Therefore, monitor for serious infusion site reactions and infusion site infections.
Patients with Parkinson’s disease have a higher risk of developing melanoma. Monitor patients for melanomas on a regular basis when using Vyalev™.
Periodic evaluation of hepatic, haematopoietic, cardiovascular and renal function is recommended during extended therapy with Vyalev™.
Vyalev™ contains hydrazine (foscarbidopa degradation product), that can be genotoxic and probably carcinogenic. The approximately median exposure of hydrazine is 0.2 mg/day, with a maximum of 0.5 mg/day. The clinical significance of this hydrazine exposure is not known.
Reduced ability to handle the delivery system can lead to complications. In such patients a caregiver should assist the patient.
A sudden or gradual worsening of bradykinesia may indicate an obstruction in the device for whatever reason and needs to be explored.
Polyneuropathy has been reported; evaluate for history/signs of and known risk factors before starting therapy.
Vyalev™ is high in sodium; considered especially in patients on a low salt diet.
Caution is needed in concomitant administration of Vyalev™ with the following medicinal products: Antihypertensives, antidepressants, COMT inhibitors, dopamine antagonists, MAO inhibitors, amantadine. Sympathomimetics may increase cardiovascular adverse events related to levodopa. Foscarbidopa is a potential inducer of CYP1A2 in vitro. Care should be taken when prescribing Vyalev™ in combination with sensitive CYP1A2 substrates (e.g. caffeine). Review section of interactions with other medicinal products in SmPC for further details about these and a complete list of interactions.
Fertility, pregnancy and lactation
Vyalev™ is not recommended during pregnancy. Breast-feeding should be discontinued during treatment with Vyalev™.
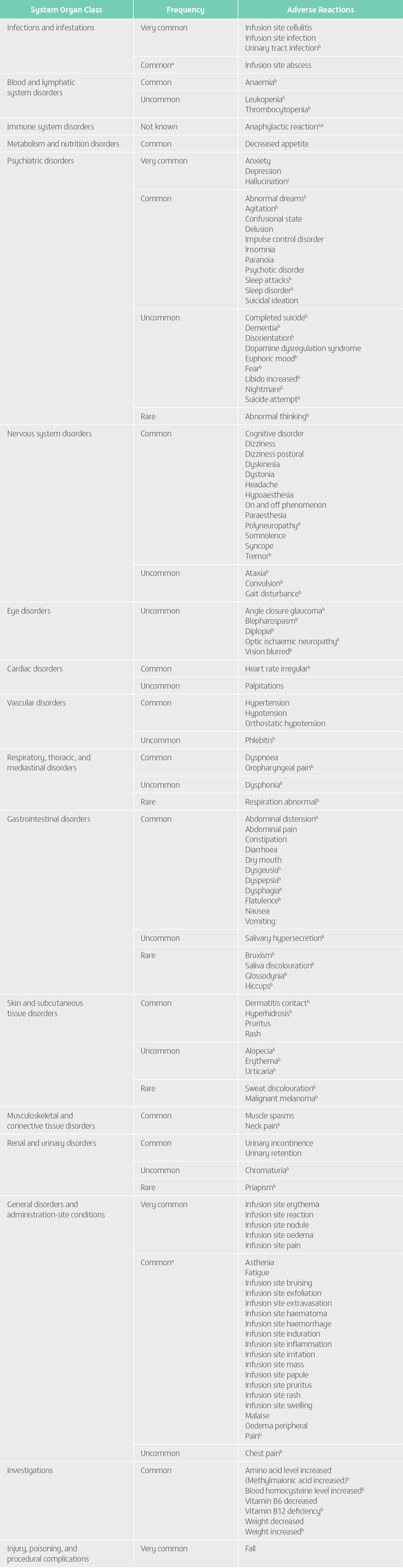
Undesirable effects
Summary of the safety profile
The most frequent adverse reactions (≥10%) reported in all Phase 3 studies in patients exposed to Vyalev™ were infusion site events (infusion site erythema, infusion site cellulitis, infusion site nodule, infusion site pain, infusion site oedema, infusion site reaction, and infusion site infection), hallucination, fall, and anxiety.
This is not a complete summary of all safety information. Please refer to your country specific product labeling for complete product prescribing and safety information.
AE-VYAL-240018