Please refer to the VYALEV™ Summary of Product Characteristics (SmPC) for complete Prescribing and Safety Information.
First and Only
A new possible course of action as the first-and-only continuous subcutaneous levodopa-based therapy for patients with advanced Parkinson’s disease.1
VYALEV™ is indicated for the treatment of advanced levodopa-responsive PD with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson’s medicinal products have not given satisfactory results.1
Continuous and Personalized
VYALEV™ provides a wide range of customizable dosing, delivered via a single infusion site and canula that can remain in place for up to 3 days, to accommodate individual patient needs.1§
VYALEV™ can be taken alone or, if necessary, with other concurrent medicinal products for PD, based on the judgment of the HCP.1
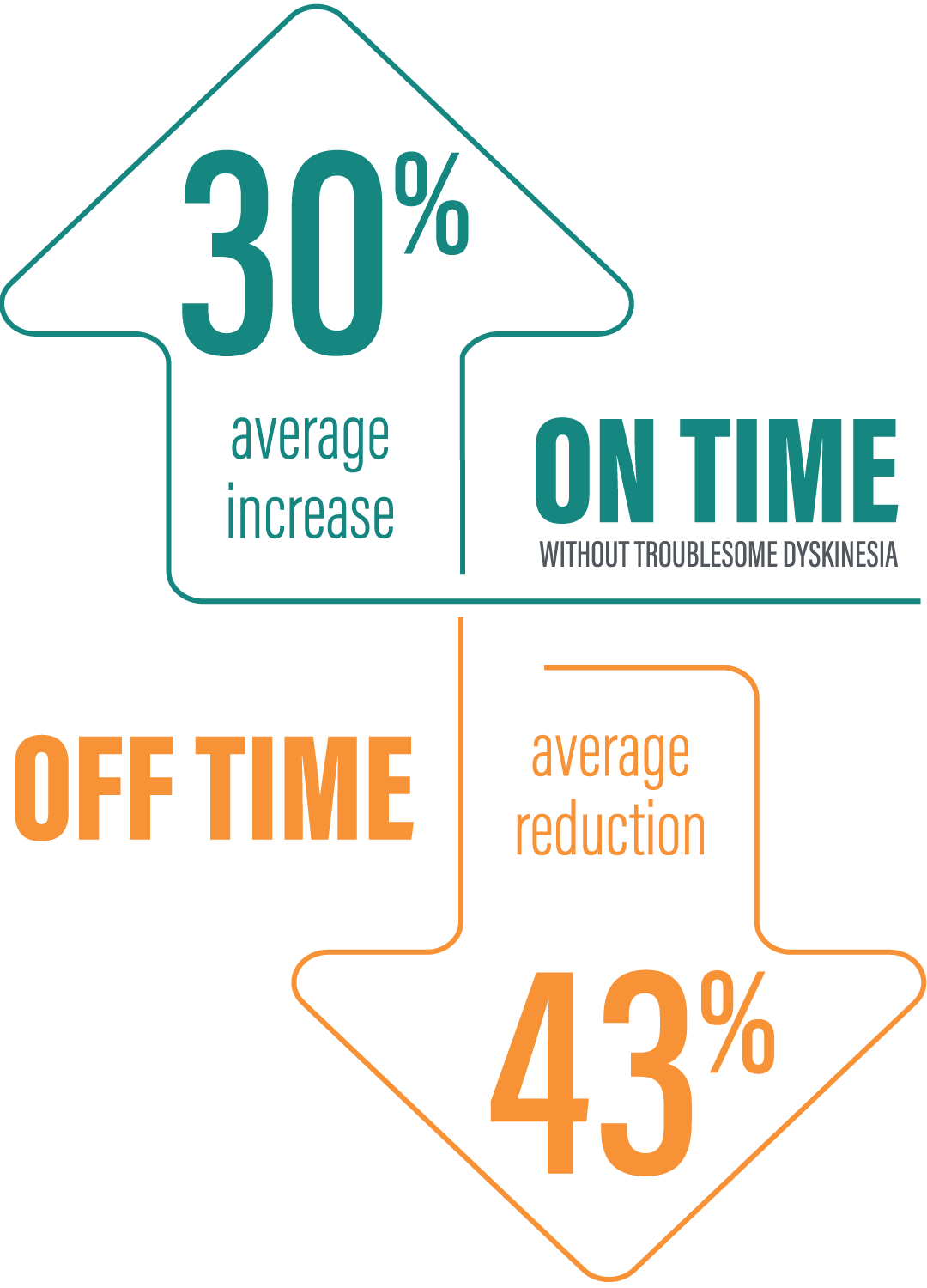
*More refers to “On” time compared to oral immediate-release (IR) levodopa/carbidopa. Patients in the VYALEV™ study experienced significant improvements compared with oral IR levodopa/carbidopa at Week 12, including “On” time without troublesome dyskinesia and “Off”time. Tap underlined link to see data.
†Patients made an entry in a PD diary upon waking and every 30 minutes during their normal waking time, and upon awakening from time asleep.1,2
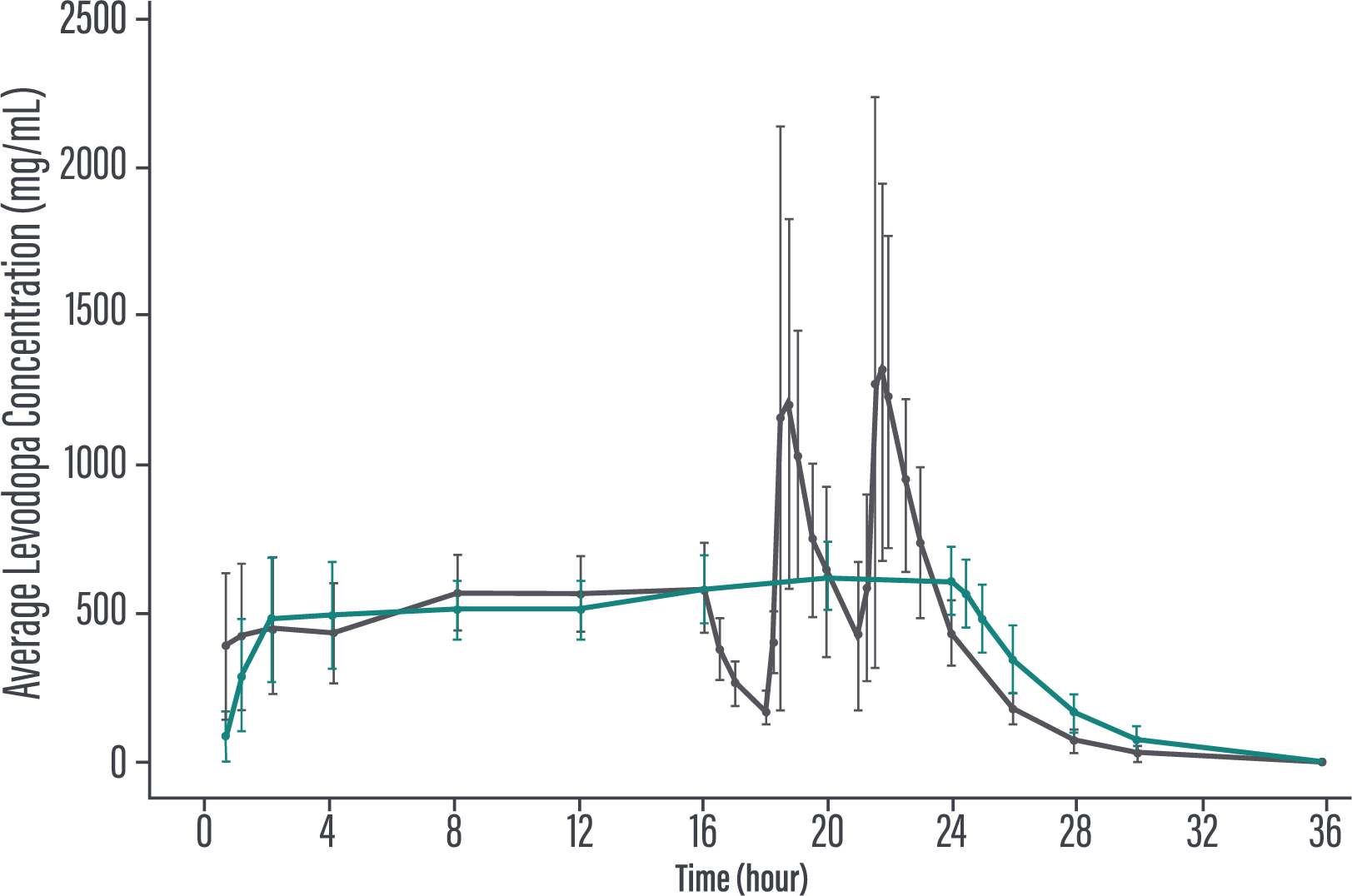
‡VYALEV™ is a levodopa-based therapy delivered subcutaneously as a 24-hour infusion.1,2
§Rotate the infusion site and use a new infusion set at least every 3 days.1 VYALEV™ allows for dosing up to 4260 mg levodopa/day with 3 programmable flow rates (base, high, and low) and an extra-dose capability. Infusion rates may be adjusted in increments as small as 0.01 mL/hour (~1.7 mg of levodopa/hour).1 For interruptions longer than 1 hour, or same infusion site/cannula used continuously for up to 3 days, a new infusion set (tubing and cannula) should be used and rotated to a different infusion site.1
HCP=healthcare professional; IR=immediate release; PD=Parkinson’s disease.
VYALEV™ is indicated for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.1
*“On” time without troublesome dyskinesia is the sum of “On” time without dyskinesia and “On” time with non‑troublesome dyskinesia.2
†Average increase in “On” time and average decrease in “Off” time with VYALEV™ are vs VYALEV™ values at baseline and were calculated by dividing the change in hours from baseline by the number of hours reported at baseline. Based on the PD diary, the average daily normalized “On” time without troublesome dyskinesia (SD) for patients on VYALEV™ was 9.20 (+/-2.42) hours at baseline and increased by 2.72 hours at Week 12 compared with an increase of 0.97 hours at Week 12 from a baseline of 9.49 (+/-2.62) hours for patients taking optimized IR levodopa/carbidopa (LS mean change). This resulted in a statistically significant improvement of 1.75 hours in patients on VYALEV™ vs oral IR LD/CD (LS mean of difference [95% CI]; P=0.0083 [0.46, 3.05]). Based on the PD diary, the average daily normalized “Off” time for patients on VYALEV™ was 6.34 (+/-2.27) hours at baseline and decreased by 2.75 hours at Week 12 compared with a decrease of 0.96 hours at Week 12 from a baseline of 5.91 (+/-1.88) hours for patients taking optimized IR levodopa/carbidopa (LS mean change). This resulted in a statistically significant improvement of 1.79 hours in patients on VYALEV™ vs oral IR LD/CD (LS mean of difference [95% CI]; P=0.0054 [-3.03, -0.54]).1
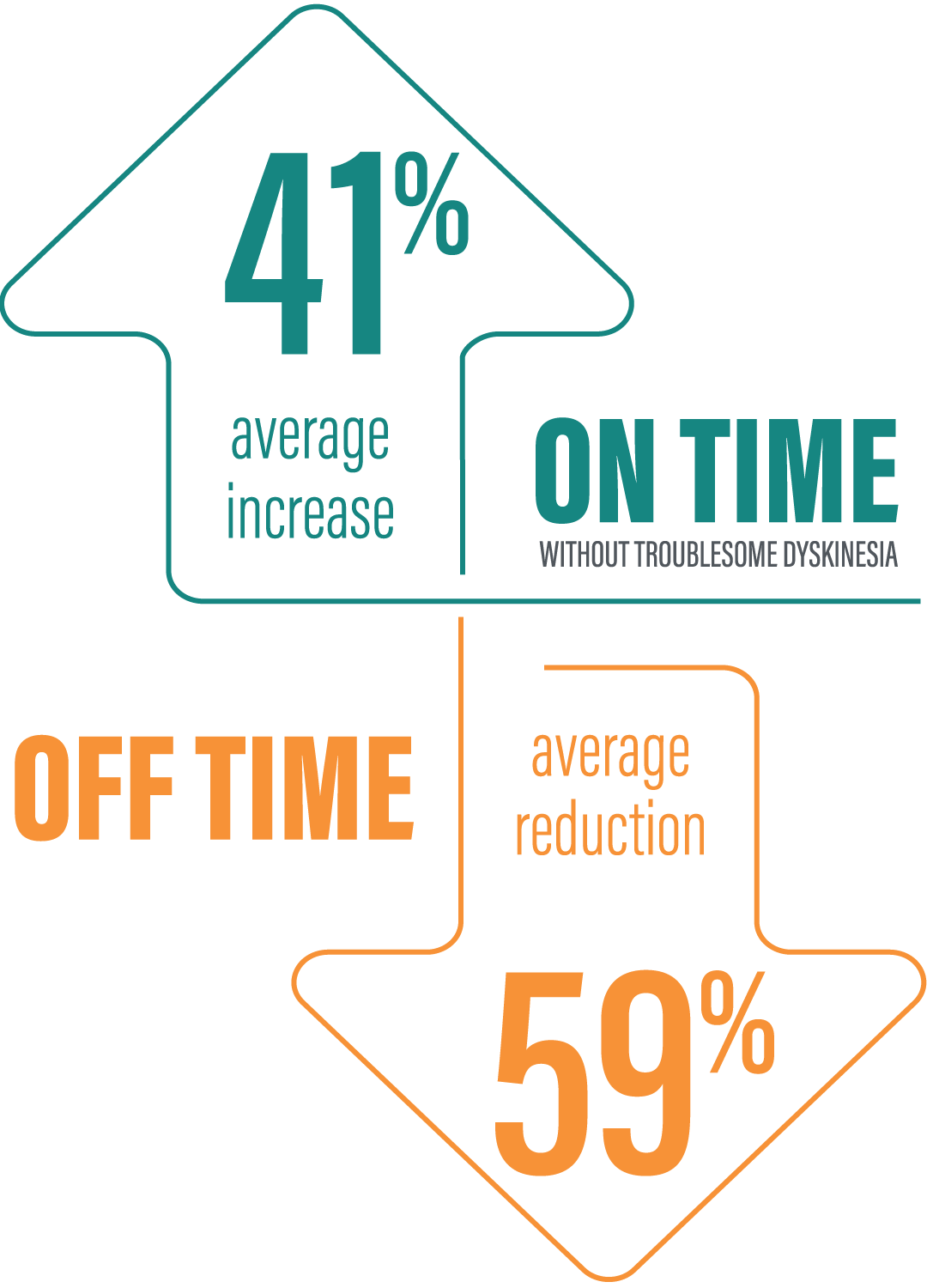
‡“On” time without troublesome dyskinesia improved by an average of 3.8 hours by Week 52 (mean 13.1 hours; n=116) compared to baseline (mean 9.1 hours; N=236) based on the PD diary. “Off” time was decreased by an average of 3.5 hours by Week 52 (mean 2.5 hours; n=116) compared to baseline (mean 5.9 hours; N=236) based on the PD diary.1,6
§Data presented reflect the third interim analysis of 52-week study results that includes 104 patients.6
CI=confidence interval; IR=immediate release; LS=least squares; SD=standard deviation; PD=Parkinson’s disease.
*Rotate the infusion site and use a new infusion set at least every three days.
Learn more about your options for starting and optimizing treatment
VYALEV™ is indicated for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson’s medicinal products have not given satisfactory results.1
Please refer to the VYALEV™ SmPC for complete Prescribing and Safety Information.
REFERENCES:
- VYALEV™ (foslevodopa/foscarbidopa solution for infusion) Summary of Product Characteristics.
- Soileau MJ, Aldred J, Budur K, et al. Lancet Neurol. 2022;21:1099–1109 (incl. suppl.).
- Rosebraugh M, Liu W, Neenan M, Facheris MF. J Parkinsons Dis. 2021;11(4):1695-1702.
- Lundqvist C. Neuropsychiatr Dis Treat. 2007;3(3):335-348.
- Tambasco N, Romoli M, Calabresi P. Curr Neuropharmacol. 2018;16(8):1239–1252.
- Aldred J, Freire-Alvarez E, Amelin AV, et al. Neurol Ther. 2023;12:1937-1958. (incl. suppl.).
- Rosebraugh M, Stodtmann S, Liu W, Facheris M. Parkinonism Relat Disord. 2022;97:68-72.
- VYALEV™ (foslevodopa/foscarbidopa solution for infusion) Patient Pump Instructions for Use.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Vyalev™ (foslevodopa and foscarbidopa solution for infusion) Indication and Summary of Important Treatment Considerations
Indication
Treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results.
Contraindications
VYALEV™ is contraindicated in patients with hypersensitivity to the active substances or to any of the excipients, narrow‑angle glaucoma, severe heart failure, acute stroke, severe cardiac arrhythmia, comedication with selective MAO type A inhibitors and nonselective MAO inhibitors, conditions contraindicated for adrenergics (e.g. pheochromocytoma, hyperthyroidism, and Cushing’s syndrome), and suspicious undiagnosed skin lesions or history of melanoma.
Select special warnings and precautions for Vyalev™
Special warnings and precautions for Vyalev™
Several warnings and precautions below are generic for levodopa and, therefore, also for Vyalev™
Not recommended for the treatment of drug-induced extrapyramidal reactions.
Caution use in patients with: severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions. History of myocardial infarction with residual atrial nodal or ventricular arrhythmias, cardiac function should be monitored during the initial dosage adjustments. Monitor all patients for the development of mental changes, depression with suicidal tendencies, and other serious mental changes. Caution with past or current psychosis and antipsychotics used concomitantly with dopamine receptor-blocking properties (observe for loss of antiparkinsonian effect). Higher frequency of hallucinations may occur with dopamine agonists and/or other dopaminergic treatments including Vyalev™. Monitor patients regularly for the development of impulse control disorders, for example Dopamine Dysregulation Syndrome (DDS). Before initiation of treatment, warn patients and caregivers of the potential risk of developing DDS. The dose of Vyalev™ may need to be adjusted downwards in order to avoid levodopa induced dyskinesias. Caution in chronic wide-angle glaucoma; monitor for intra-ocular pressure changes. Vyalev™ may induce orthostatic hypotension and should be given cautiously in patients taking other medicinal products that may cause orthostatic hypotension. Concomitant use of selegiline and levodopa/carbidopa has been associated with serious orthostatic hypotension. Levodopa may induce somnolence and sudden sleep: caution should be exercised when driving and operating machines. Risk of symptoms resembling Neuroleptic Malignant Syndrome following abrupt dose reduction or discontinuation.
Infusion site events (see section 4.8) have been reported in patients receiving Vyalev™. Follow aseptic techniques and frequently rotate the infusion site to reduce the risk. In clinical studies, few patients who reported infusion site reactions also experienced infusion site infections. Therefore, monitor for serious infusion site reactions and infusion site infections.
Patients with Parkinson’s disease have a higher risk of developing melanoma. Monitor patients for melanomas on a regular basis when using Vyalev™.
Periodic evaluation of hepatic, haematopoietic, cardiovascular and renal function is recommended during extended therapy with Vyalev™.
Vyalev™ contains hydrazine (foscarbidopa degradation product), that can be genotoxic and probably carcinogenic. The approximately median exposure of hydrazine is 0.2 mg/day, with a maximum of 0.5 mg/day. The clinical significance of this hydrazine exposure is not known.
Reduced ability to handle the delivery system can lead to complications. In such patients a caregiver should assist the patient.
A sudden or gradual worsening of bradykinesia may indicate an obstruction in the device for whatever reason and needs to be explored.
Polyneuropathy has been reported; evaluate for history/signs of and known risk factors before starting therapy.
Vyalev™ is high in sodium; considered especially in patients on a low salt diet.
Caution is needed in concomitant administration of Vyalev™ with the following medicinal products: Antihypertensives, antidepressants, COMT inhibitors, dopamine antagonists, MAO inhibitors, amantadine. Sympathomimetics may increase cardiovascular adverse events related to levodopa. Foscarbidopa is a potential inducer of CYP1A2 in vitro. Care should be taken when prescribing Vyalev™ in combination with sensitive CYP1A2 substrates (e.g. caffeine). Review section of interactions with other medicinal products in SmPC for further details about these and a complete list of interactions.
Fertility, pregnancy and lactation
Vyalev™ is not recommended during pregnancy. Breast-feeding should be discontinued during treatment with Vyalev™.
Undesirable effects
Summary of the safety profile
The most frequent adverse reactions (≥10%) reported in all Phase 3 studies in patients exposed to Vyalev™ were infusion site events (infusion site erythema, infusion site cellulitis, infusion site nodule, infusion site pain, infusion site oedema, infusion site reaction, and infusion site infection), hallucination, fall, and anxiety.
This is not a complete summary of all safety information. Please refer to your country specific product labeling for complete product prescribing and safety information.
AE-VYAL-240018