This promotional material is intended for UK Healthcare Professionals (HCPs) experienced in the diagnosis and management of Parkinson’s disease only. Adverse event reporting can be found below
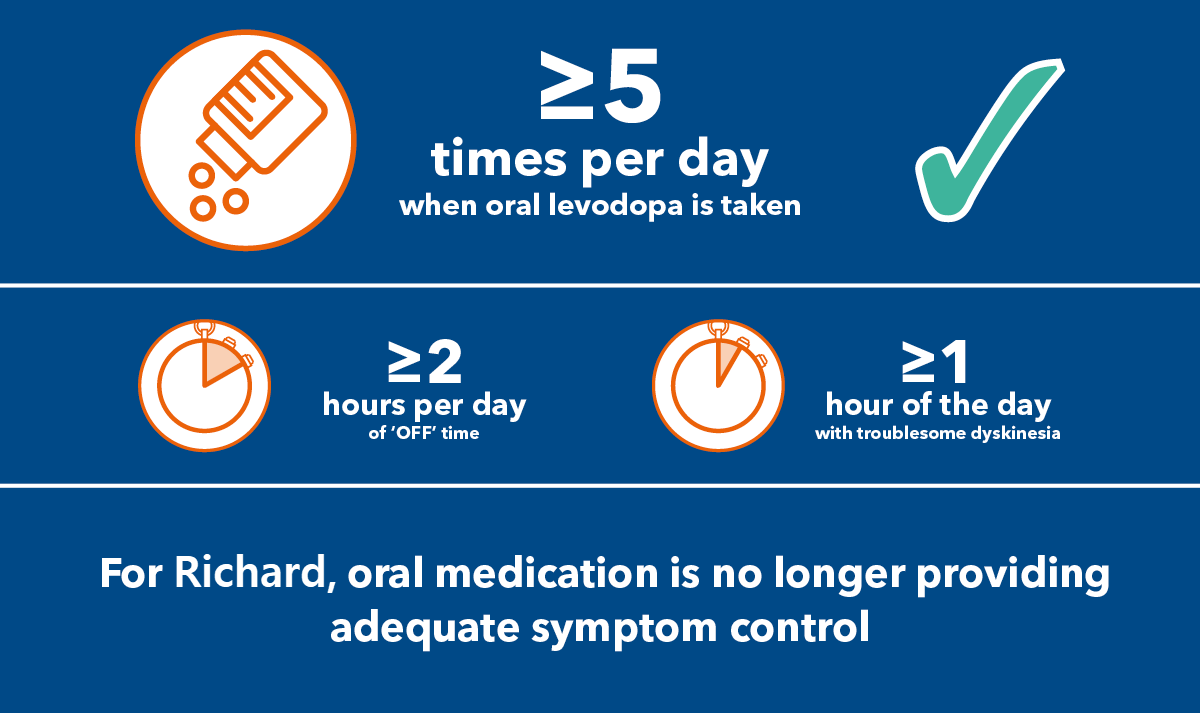
Richard experiences uncontrolled PD. His dosing frequency has exceeded 5 doses per day, so he's reached a threshold to be considered for a non-oral therapy.
Meet Richard
62 years old
Onset of PD at 56
Married to Lena, has four grown children
and 12 grandchildren
Former tax advisor
Symptomology
'ON' time during his waking day limited to 4 hours
1 hour of 'OFF' time
Non-troublesome dyskinesia
History
Has tried most anti-Parkinson's oral medications available
PD not well controlled
8 doses of oral levodopa a day
Impact of motor symptoms
Trouble dressing
Fatigue
Difficulty chewing and swallowing
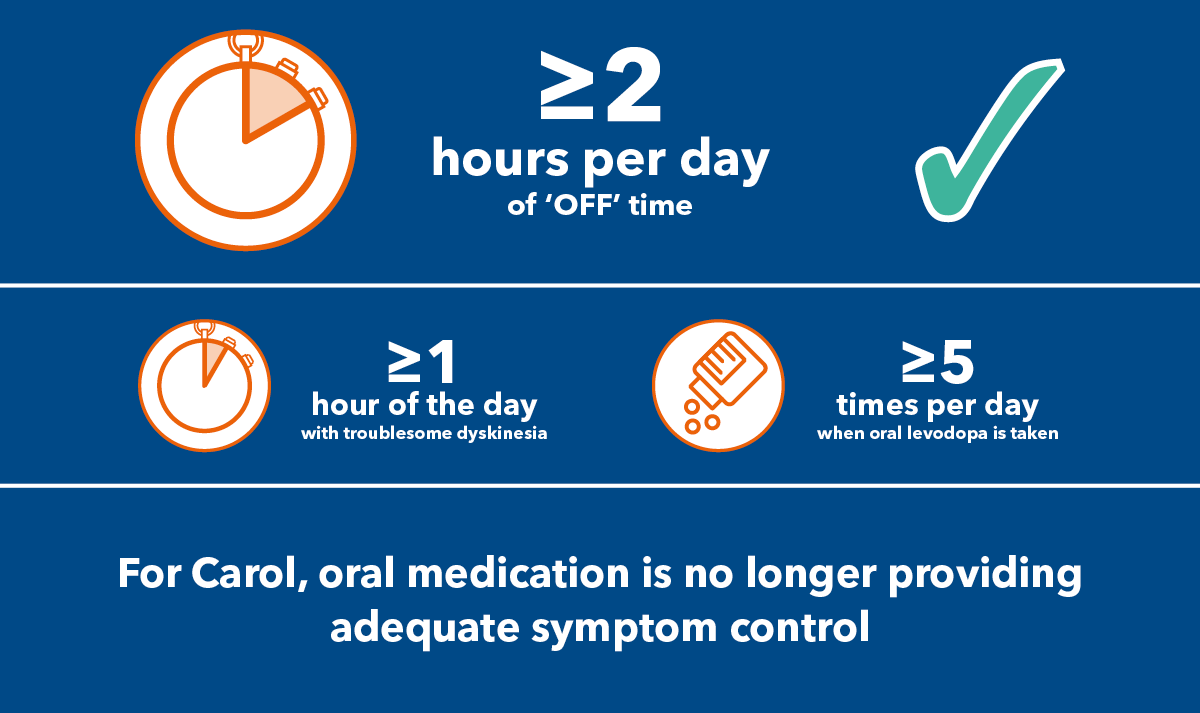
As well as her 4 doses of levodopa, Carol takes a COMT inhibitor. However, control is increasingly wearing-off and she experiences at least 3 hours of 'OFF' time every day. She's reached a threshold to be considered for a non-oral therapy.
Meet Carol
59 years old
Onset of PD at 53
Lives with husband, has two grown daughters
Former professional artist
Symptomology
Increasing problem with wearing-off
Experiences non-troublesome dyskinesias
3 hours of 'OFF' time a day
History
Good response to oral levodopa
PD not well controlled
4 doses of oral levodopa a day
COMT inhibitor
Impact of motor symptoms
Can no longer paint/draw
Trouble getting out of bed
Fatigue
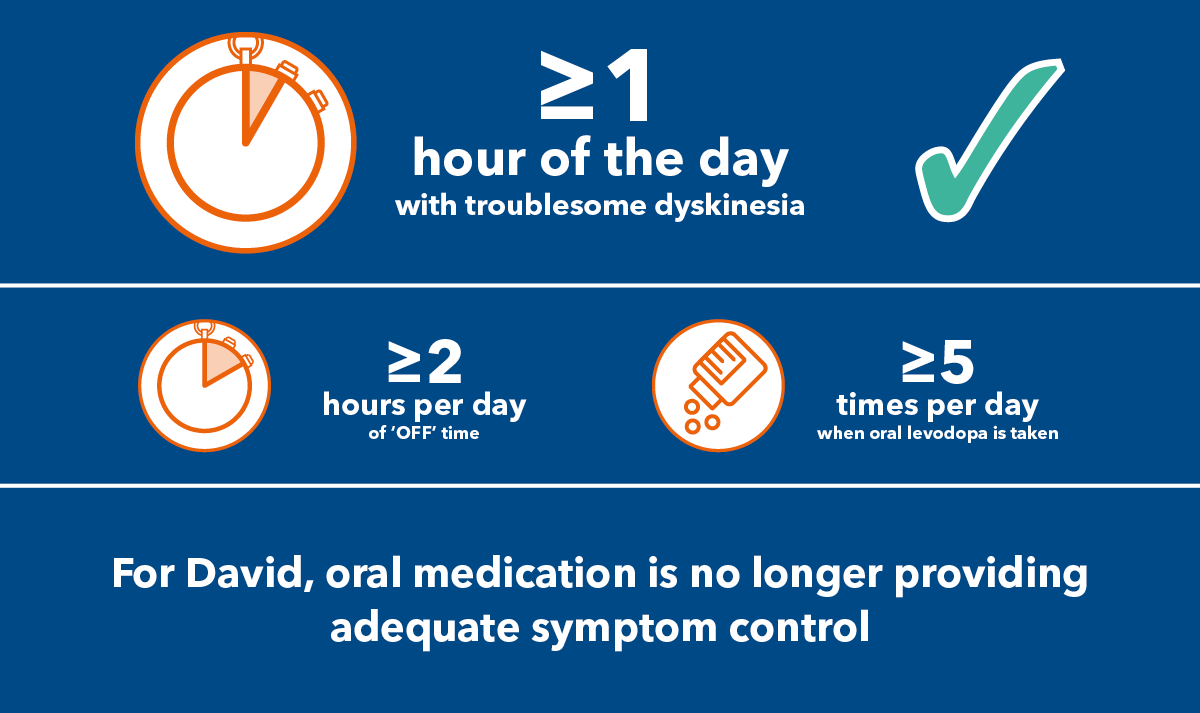
David's PD is not well controlled. The dose of levodopa has been increased, but there are still unpredictable fluctuations of motor symptoms. David experiences 3 hours of troublesome dyskinesia every day
Meet David
75 years old
Onset of PD at 66
Loves to go fishing with wife Jenny
Symptomology
Increased levodopa diminished 'OFF' time
Unpredictable fluctuations of motor symptoms
Prescribed amantadine
3 hours of troublesome dyskinesia
History
Responding to MAOBi
PD not well controlled
Impact of motor symptoms
Dressing
Moving around the house
Struggling to continue fishing
COMT=catechol-O-methyltransferase; MAOBi=monoamine oxidase B inhibitor.
Learn about the progression of PD
Stay connected
Keep up to date with future resources, support, and guidance to help you manage your patients with PD by filling out your details below and joining the mailing list.
Please only fill out your details if you are a UK registered healthcare professional.
1. DUODOPA (levodopa/carbidopa intestinal gel) SmPC.
2. PRODUODOPA (foslevodopa/foscarbidopa solution for infusion) SmPC.
By clicking the links above you will leave the AbbVie Pro website and be taken to the eMC PI portal website
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
UK-PRODD-240198. Date of preparation: December 2024