This promotional material is intended for UK Healthcare Professionals (HCPs) experienced in the diagnosis and management of Parkinson’s disease only. Adverse event reporting can be found below
Patients with PD who experience ‘OFF’ time may have difficulty performing everyday activities and communicating effectively5,8-10
In an online US survey of 2,110 patients with PD, that assessed their lived experience of ‘OFF’ periods:
Nearly a quarter of their waking hours were spent in ‘OFF’ state (mean disease duration 6.1 years), which comprised both motor and non-motor symptoms.8
Patients with PD reported a wide range of motor symptoms during their ‘OFF’ times, with some describing these symptoms as ‘constant tremors’, being ‘frozen in place’ and ‘moving in slow motion’.8
Findings from 3 studies whose aims all included exploring the impact of ‘OFF’ periods on PD patients (An online survey of 305 patients exploring the impact of ‘OFF’ time on health related quality of life and daily functioning in people with PD, a scoping review that included 23 studies that evaluated the impact or experience of ‘OFF’ periods in PD patients, and an epidemiological survey of a multicentre, observational cross-sectional study of 617 patients assessing the frequency of wearing ‘OFF’ in patients with PD and its impact on quality of life).
‘OFF’ times made it impossible for them to leave the house on their own and affected their ability to communicate effectively.5,9,10
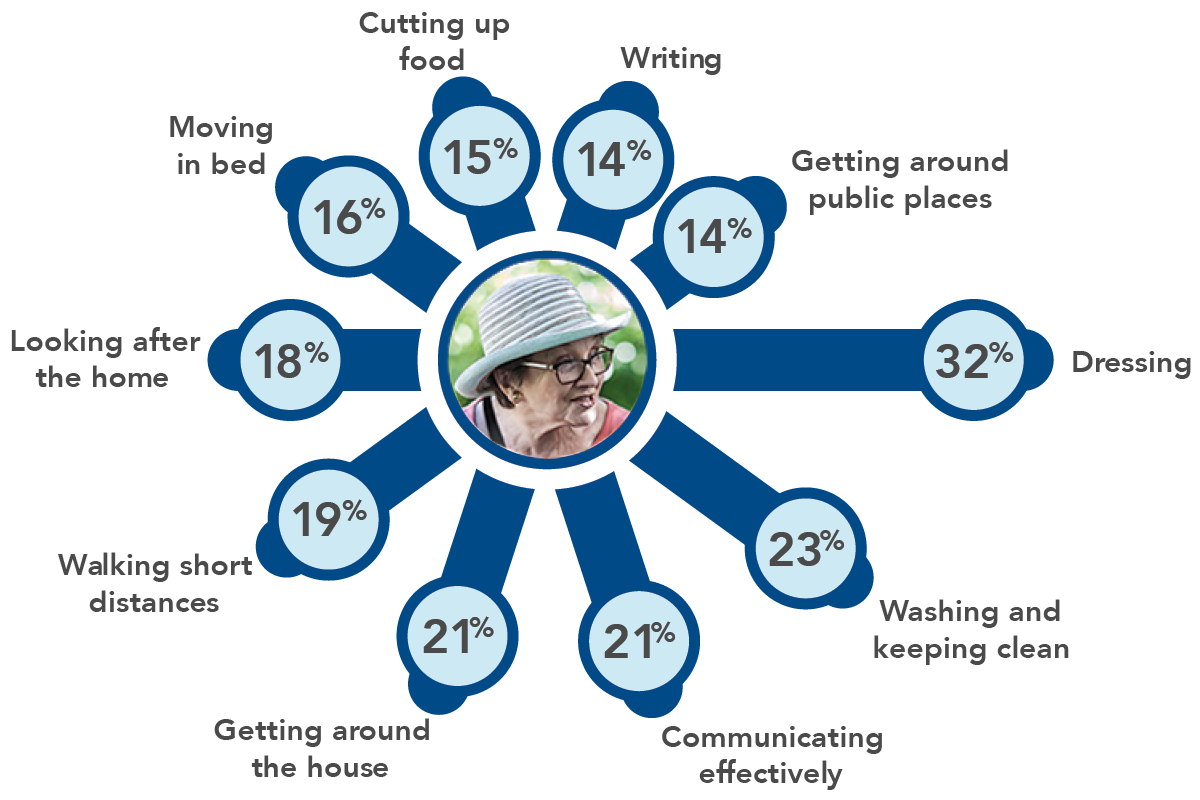
The patient perspective: Effect on functioning and ability to undertake usual activities
What is most bothersome about ‘OFF’ time? Results from an online survey of patients with Complex/Advanced PD experiencing motor fluctuations/wearing off with levodopa5
Activities rated as most bothersome due to being most limited during ‘OFF’ time (N=305)5
Adapted from Kerr C et al, 2016.
Approximately 75% of care is provided by a
spouse/partner for 10 years or more12
Discover why earlier patient assessment for non-oral therapies is important
Stay connected
Keep up to date with future resources, support, and guidance to help you manage your patients with PD by filling out your details below and joining the mailing list.
Please only fill out your details if you are a UK registered healthcare professional.
1. DUODOPA (levodopa/carbidopa intestinal gel) SmPC.
2. PRODUODOPA (foslevodopa/foscarbidopa solution for infusion) SmPC.
3. Pahwa R, et al. Parkinsonism Relat Disord. 2019; 60: 118–25.
4. Odin P, et al. Parkinsonism Relat Disord. 2015; 21: 1133–44.
5. Kerr C, et al. Qual Life Res. 2016; 25(6): 1505–15.
6. Haahr A, et al. J Adv Nurs. 2011; 67(2): 408–17.
7. Antonini A, et al. Neurol Ther. 2022; 11(1): 303–18.
8. Mantri S, et al. J Patient Cent Res Rev. 2021; 8(3): 232–8.
9. Rastgardani T, et al. Mov Disord Clin Pract. 2018; 5(5): 461–70.
10. Stocchi F, et al. Parkinsonism Relat Disord. 2014; 20(2): 204–11.
11. Smolensky L, et al. Sci Data. 2020; 7(1): 67.
12. Hassan A, et al. Parkinsonism Relat Disord 2012; Suppl 3: S10-14.
By clicking the links above you will leave the AbbVie Pro website and be taken to the eMC PI portal website
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or via the MHRA Yellow Card app, available in the Google Play or Apple App Stores.
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
UK-PRODD-240195. Date of preparation: December 2024