The Patient Experience: Quality of Life
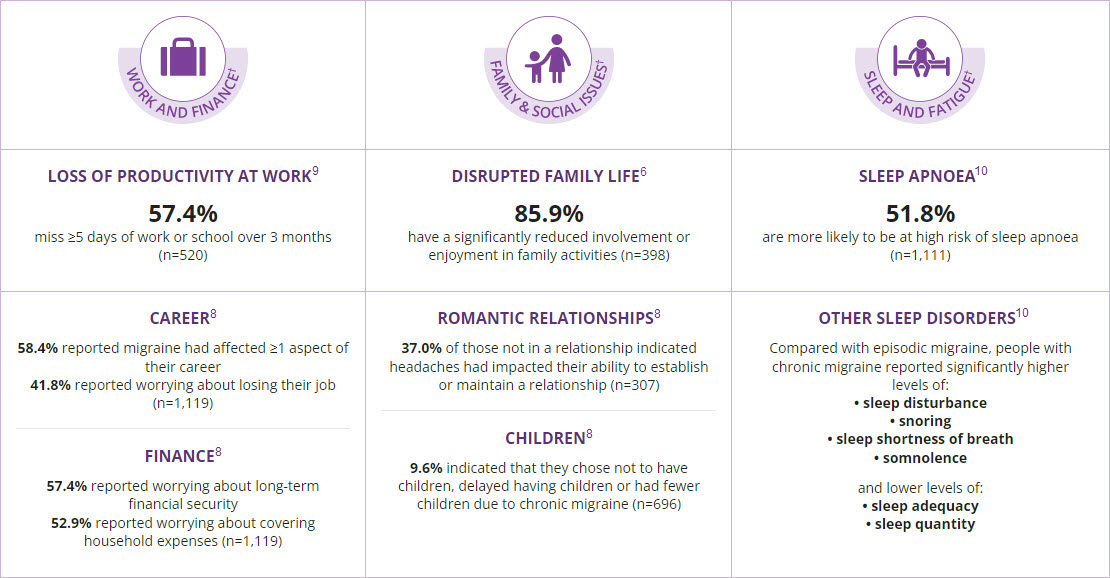
Migraine is associated with a substantial personal burden, resulting from headache-related disability, a high rate of comorbidities, reduced health-related quality of life and a concomitant financial burden.6,7 Migraine can also affect work productivity, interpersonal dynamics and psychological health and wellbeing, both for the patient and for their family.8 Unsurprisingly, it has been shown that this impact is greatest in families of people with chronic migraine.8
The personal burden of chronic migraine
†data for patients with chronic migraine.
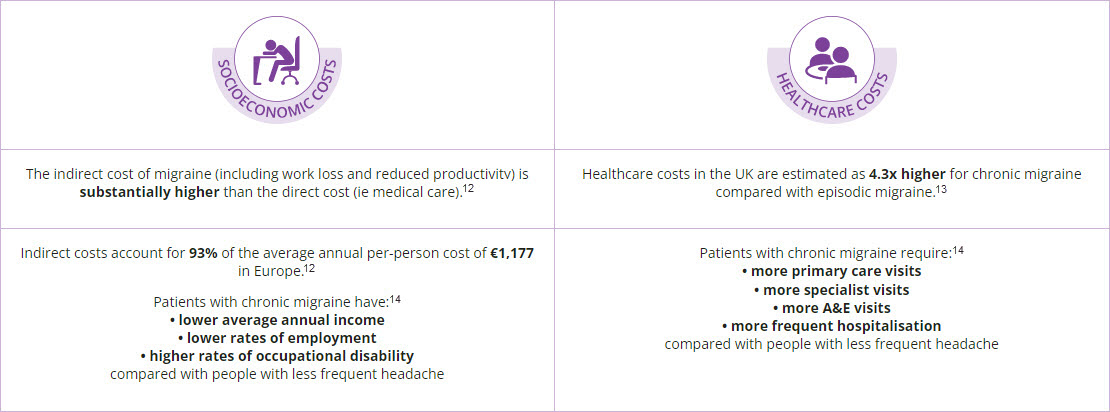
The societal burden of chronic migraine
The economic burden of the disease reflects the higher disease severity and associated comorbidities of chronic migraine compared with episodic migraine.11
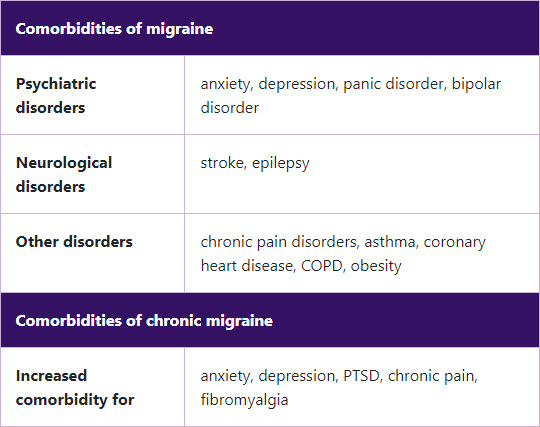
Common comorbidities in patients with chronic migraine9,14
It is common for patients to experience anticipatory anxiety of their next migraine attack during a pain-free period. This can also encourage overuse of analgesic medication.12
Chronic migraine is a complex neurological disease requiring appropriate effective management - including treatment or referrals to improve patient outcomes2,15,16
CM: chronic migraine; COPD: chronic obstructive pulmonary disease; PTSD: post-traumatic stress disorder.
Please refer to the BOTOX® Summary of Product Characteristics for further information on adverse events, contraindications and special warnings and precautions for use.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk
Adverse events should also be reported to AbbVie on GBPV@abbvie.com
Date of preparation: March 2024. UK-BCM-240043.