TEPKINLY DOSING AND ADMINISTRATION
The potential for ongoing tumour suppression and surveillance with a monthly maintenance dosing schedule
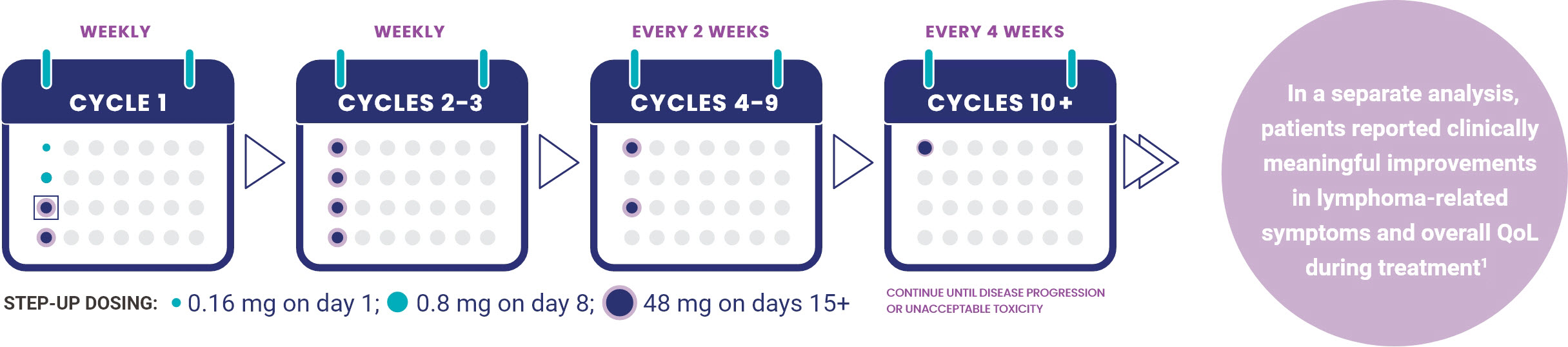
0.16 mg is a priming dose, 0.8 mg is an intermediate dose, and 48 mg is a full dose
- TEPKINLY should be administered until disease progression or unacceptable toxicity
- TEPKINLY should be administered to adequately hydrated patients
- At least 1 dose of tocilizumab for use in the event of CRS should be available prior to TEPKINLY for cycle 1. Access to an additional dose of tocilizumab within 8 hours of use of the previous tocilizumab dose should be available
Patients should be monitored for signs and symptoms of CRS and/or immune effector cell-associated neurotoxicity syndrome (ICANS) following epcoritamab administration. Patients should be hospitalised for 24 hours after administration of the Cycle 1 Day 15 dose of 48 mg to monitor for signs and symptoms of CRS and/or ICANS. Patients should be counselled on the signs and symptoms associated with CRS and ICANS and on seeking immediate medical attention should signs or symptoms occur at any time.
A 1-mL SC injection*
- Dosing every 2 weeks after cycle 3 (28-day cycle)
- Dosing every 4 weeks after cycle 10
*“Quick” is corroborated by the significantly faster administration with subcutaneous dosing as compared with IV alternatives.
The TEPKINLY EPCORE™ NHL-1 clinical trial was designed to mitigate T-cell engager–induced cytokine release
- TEPKINLY should be administered to adequately hydrated patients
- Patients should be monitored for signs and symptoms of CRS and/or ICANS following TEPKINLY administration
- Patients should be hospitalised for 24 hours after administration of the cycle 1, day 15 dose of 48 mg to monitor for signs and symptoms of CRS and/or ICANS
- Patients should be counselled on the signs and symptoms associated with CRS and ICANS and on seeking immediate medical attention should signs or symptoms occur at any time
Premedication can be taken at home orally prior to weekly TEPKINLY administration
Download the Dosing and Administration Guide for more information on how TEPKINLY is administered.
References:
- Tepkinly prescribing information
IL-EPCOR-240017. Date of preparation: July 2024.