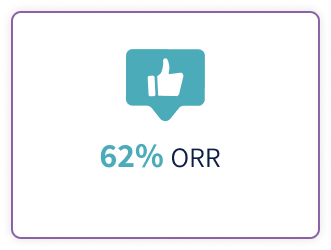
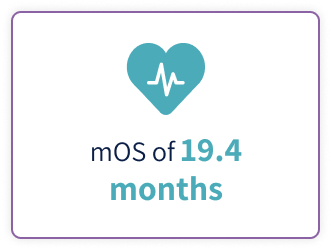
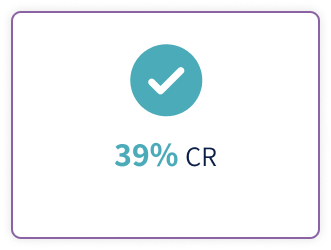
Tepkinly (epcoritamab 48mg\0.8 ml, 4mg\0.8 ml) is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from indolent lymphoma, and high-grade B cell lymphoma after two or more lines of systemic therapy1.
MOA
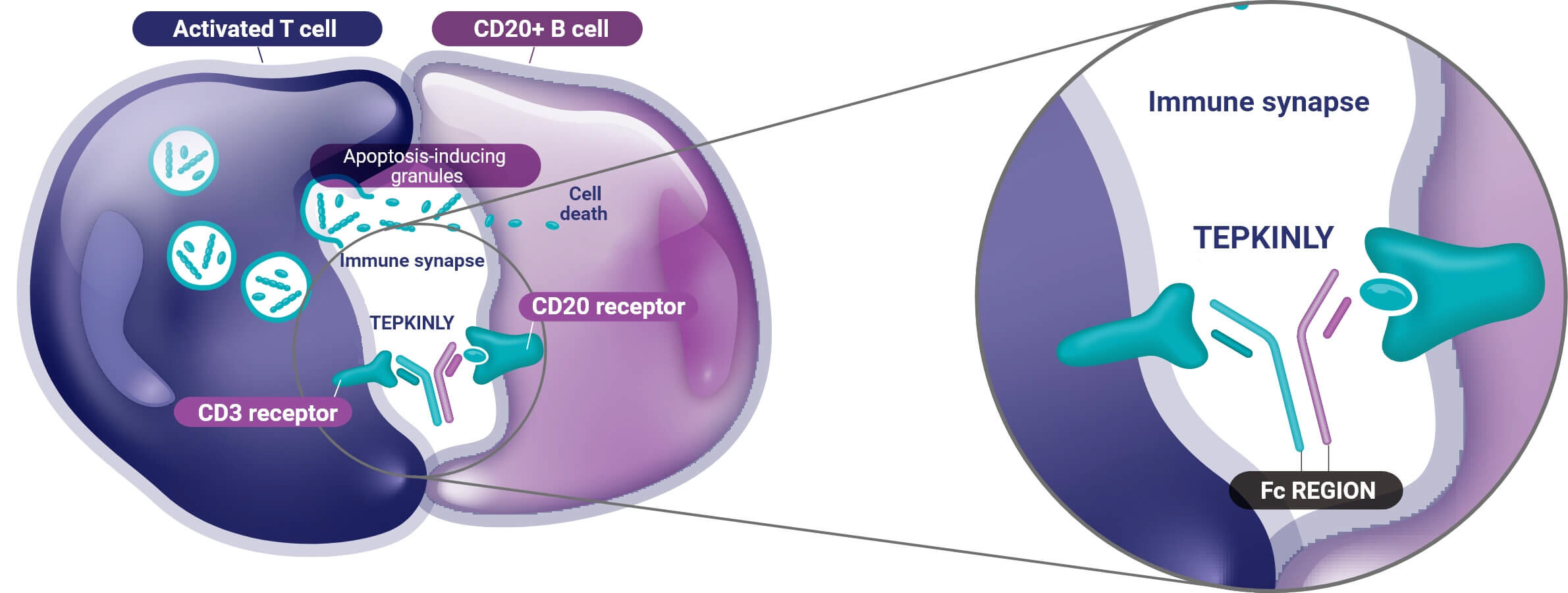
A subcutaneous T-cell engaging CD3xCD20 bispecific antibody
Specifically engineered for high-specificity binding and minimal off-target effects
By binding CD20-expressing B cells and CD3-expressing T cells, TEPKINLY induces specific T-cell activation and T-cell–mediated killing of the CD20-expressing cancer cells.
Efficient T-cell–mediated cytotoxicity was achieved even with very low levels of CD20 expression.
In preclinical studies, TEPKINLY demonstrated specific and highly potent antitumour activity in vitro and in vivo.
References:
- Tepkinly prescribing information
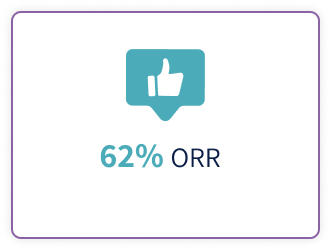
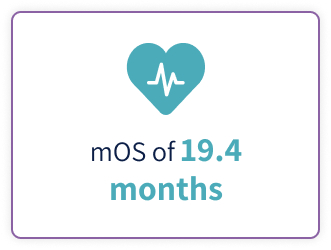
- Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238-2247. doi:10.1200/JCO.22.01725
- Jurczak W, Ghesquieres H, Karimi Y, et al. P1118: longer follow-up from the pivotal EPCORE NHL-1 trial reaffirms subcutaneous epcoritamab induces deep, durable complete remissions in patients with relapsed/refractory large B-cell lymphoma. Hemasphere. 2023;7(suppl):e081065c. doi:10.1097/ 01.HS9.0000971368.08106.5c
IL-EPCOR-240017. Date of preparation: July 2024.