PRODUODOPA Has a Well-studied Safety Profile.1
Produodopa Was Generally Safe.
The Majority of AEs Reported Were Non-Serious and Mild/Moderate.
AE: Adverse Event; TEAE: Treatment Emergent Adverse Event.
Adverse reactions reported in all Phase 3 studies in patients exposed to PRODUODOPA (379 patients with total exposure of 414.3 person-years, 230 subjects exposed for ≥6 months, 204 subjects exposed for ≥12 months) or data from DUODOPA (levodopa/carbidopa intestinal gel) based on treatment emergent frequencies, regardless of causality assigned.1
| System Organ Class | Frequency | Adverse Reactions |
Infections and infestations | Very common (≥1/10) | Infusion site cellulitis Infusion site infection Urinary tract infection† |
Common (≥1/100 to <1/10)* | Infusion site abscess | |
| Blood and lymphatic system disorders | Common (≥1/100 to <1/10) | Anaemia† |
| Uncommon (≥1/1,000 to <1/100) | Leukopenia† Thrombocytopenia† | |
| Immune system disorder | Not known | Anaphylactic reaction†‖ |
Metabolism and nutrition disorders | Common (≥1/100 to <1/10) | Decreased appetite |
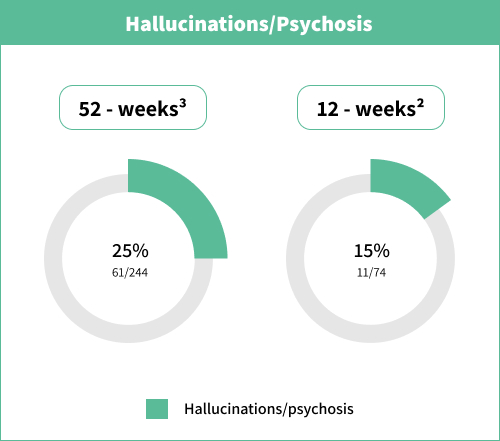
Psychiatric disorders | Very common (≥1/10) | Anxiety Depression Hallucination‡ |
| Common (≥1/100 to <1/10) | Abnormal dreams† Agitation† Confusional state Delusion Impulse control disorder Insomnia Paranoia Psychotic disorder Sleep attacks† Sleep disorder† Suicidal ideation | |
| Uncommon (≥1/1,000 to <1/100) | Completed suicide† Dementia† Disorientation† Dopamine dysregulation syndrome Euphoric mood† Fear† Libido increased† Nightmare† Suicide attempt† | |
| Rare (≥1/10,000 to <1/1,000) | Abnormal thinking† | |
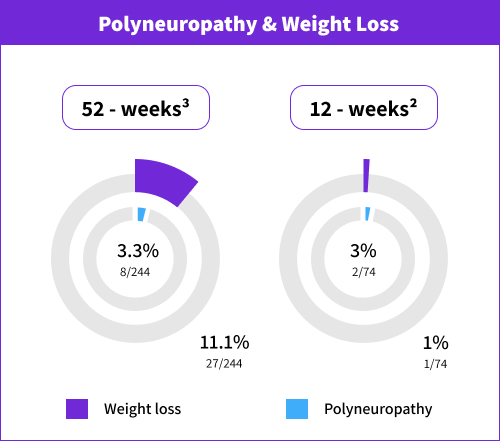
Nervous system disorders | Common (≥1/100 to <1/10) | Cognitive disorder Dizziness Dizziness postural Dyskinesia Dystonia Headache Hypoaesthesia On and off phenomenon Paraesthesia Polyneuropathy§ Somnolence Syncope Tremor† |
| Uncommon (≥1/1,000 to <1/100) | Ataxia† Convulsion† Gait disturbance† | |
Eye disorders | Uncommon (≥1/1,000 to <1/100) | Angle closure glaucoma† |
Cardiac disorders | Common (≥1/100 to <1/10) | Heart rate irregular† |
Uncommon (≥1/1,000 to <1/100) | Palpitations | |
Vascular disorders | Common (≥1/100 to <1/10) | Hypertension |
Uncommon (≥1/1,000 to <1/100) | Phlebitis† | |
Respiratory, thoracic, and mediastinal disorders | Common (≥1/100 to <1/10) | Dyspnoea Oropharyngeal pain† |
Uncommon (≥1/1,000 to <1/100) | Dysphonia† | |
Rare (≥1/10,000 to <1/1,000) | Respiration abnormal† | |
Gastrointestinal disorders | Common (≥1/100 to <1/10) | Abdominal distension† Abdominal pain Constipation Diarrhoea Dry mouth Dysgeusia† Dyspepsia† Dysphagia† Flatulence† Nausea Vomiting |
| Uncommon (≥1/1,000 to <1/100) | Salivary hypersecretion† | |
| Rare (≥1/10,000 to <1/1,000) | Bruxism† Saliva discolouration† Glossodynia† Hiccups† | |
Skin and subcutaneous tissue disorders | Common (≥1/100 to <1/10) | Dermatitis contact† Hyperhidrosis† Pruritus Rash |
| Uncommon (≥1/1,000 to <1/100) | Alopecia† Erythema† Urticaria† | |
| Rare (≥1/10,000 to <1/1,000) | Sweat discolouration† Malignant melanoma† | |
Musculoskeletal and connective tissue disorders | Common (≥1/100 to <1/10) | Muscle spasms |
Renal and urinary disorders | Common (≥1/100 to <1/10) | Urinary incontinence Urinary retention |
| Uncommon (≥1/1,000 to <1/100) | Chromaturia† | |
| Rare (≥1/10,000 to <1/1,000) | Priapism† | |
General disorders and administration site conditions | Very common (≥1/10) | Infusion site erythema Infusion site reaction Infusion site nodule Infusion site oedema Infusion site pain |
| Common (≥1/100 to <1/10)* | Asthenia Fatigue Infusion site bruising Infusion site exfoliation Infusion site extravasation Infusion site haematoma Infusion site haemorrhage Infusion site induration Infusion site inflammation Infusion site irritation Infusion site mass Infusion site papule Infusion site pruritus Infusion site rash Infusion site swelling Malaise Oedema peripheral Pain† | |
| Uncommon (≥1/1,000 to <1/100) | Chest pain | |
Investigations | Common (≥1/100 to <1/10) | Amino acid level increased (Methylmalonic acid increased)† |
Injury, poisoning, and procedural complications | Very common (≥1/10) | Fall |
*Common adverse reactions pertaining to infusion site events included if ≥2%. †These adverse reactions were identified with DUODOPA intestinal gel as drug-related events. However, these events were not considered adverse reactions for PRODUODOPA. ‡Hallucination includes hallucination, hallucination visual, hallucination auditory, hallucination olfactory, hallucinations tactile, and hallucinations mixed. §Polyneuropathy includes neuropathy peripheral, polyneuropathy, decreased vibratory sense, peripheral sensory neuropathy, sensory disturbance, and sensory loss. ‖Based on post-marketing data.
References
- PRODUODOPA (foslevodopa/foscarbidopa solution for infusion) prescribing information.
- Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
- Aldred J, et al. Neurol Ther. 2023 Aug 26. doi: 10.1007/5. s40120-023-00533.
IL-PRODD-240009. Date of preparation: July 2024.







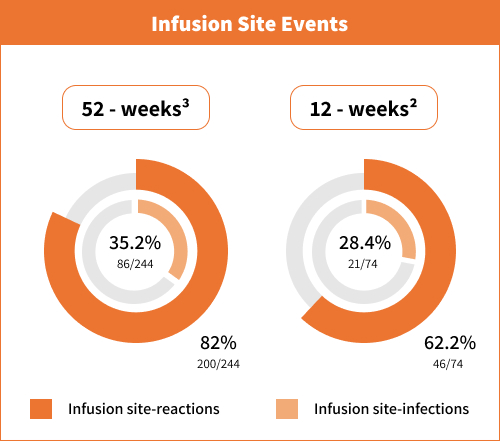
 Infusion site events (infusion site erythema, infusion site cellulitis, infusion site nodule,
Infusion site events (infusion site erythema, infusion site cellulitis, infusion site nodule, infusion site pain, infusion site oedema, infusion site reaction, and infusion site infection).
infusion site pain, infusion site oedema, infusion site reaction, and infusion site infection).