Injection Guides
Chronic Migraine -Injection Workbook
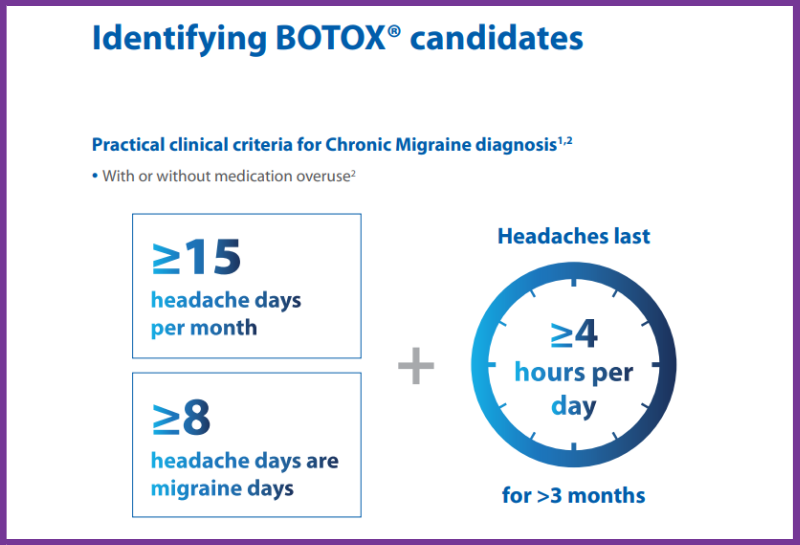
Guidance for identifying Botox candidates, the injection procedure, and discussing treatment with patients.
To view the workbook click here
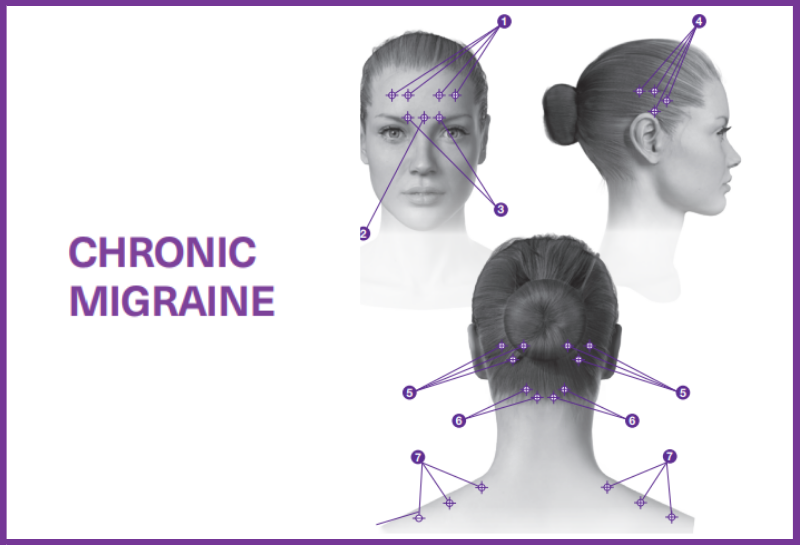
Botox for Chronic Migraine – PREEMT Protocol
Based on a publication by Blumenfeld et al 2017 (INSIGHTS into the functional anatomy behind the PREEMPT injection paradigm)
To view PREEMT protocol click here
References:
1. BOTOX® PI 2. Khalil M, et al. J Headache Pain. 2014;15:54. 3. Lee MJ, et al. J Neurol Sci. 2016;363:51–54. 4. Negro A, et al. Springerplus. 2015;4:826. 5. Negro A, et al. J Headache Pain. 2016;17:1. 6. Lia C, et al. Neurol Sci. 2014;35(Suppl 1):175–176. 7. Kollewe K, et al. J Neural Transm. 2016;123(5):533–540. 8. Cernuda-Morollon E, et al. Pain. 2015;156(5):820–824. 9. Aicua-Rapun I, et al. J Headache Pain. 2016;17:112. 10. Demiryurek BE, et al. Neurol Sci. 2016;37:1779–1784. 11. Vikelis M, et al. J Headache Pain. 2016;17(1):84. 12. Santoro A, et al. Neurol Sci 41, 1809–1820 (2020) 13. Blumenfeld A, et al. Headache. 2010;50(9):1406–1418. 14. Data on file, Allergan, 2017; InCrowd Physician Survey
IL-BTX-230012 | May 2024