Safety Profile1
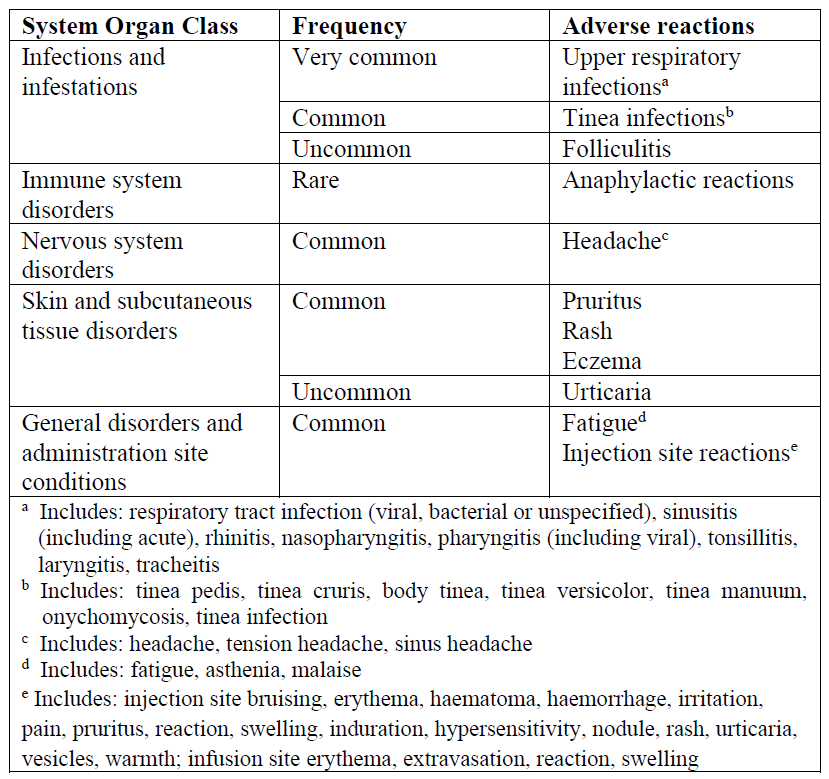
Most Frequent Adverse Reactions
The most frequent adverse reactions were upper respiratory infections
Please refer to prescribing information for more information.
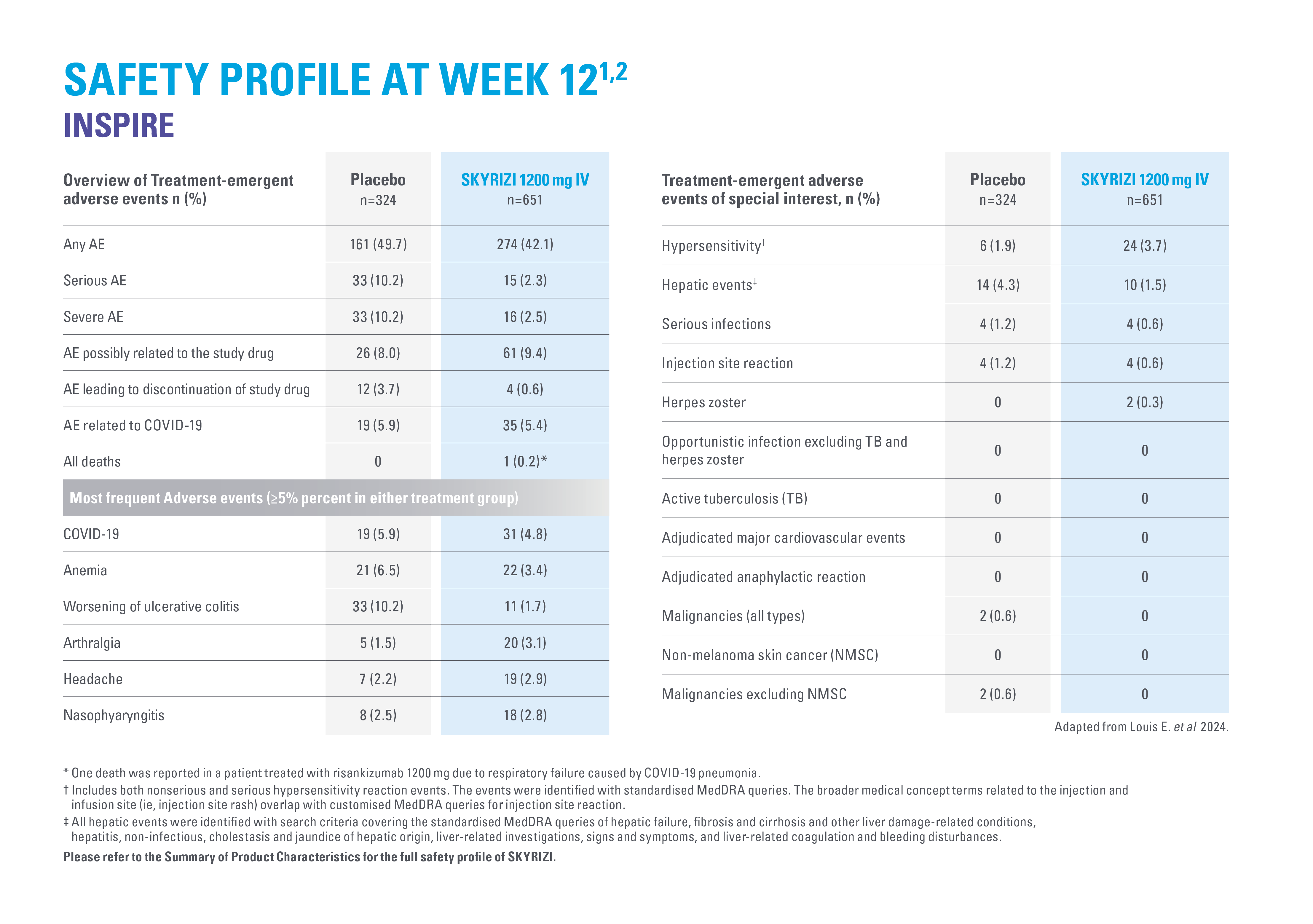
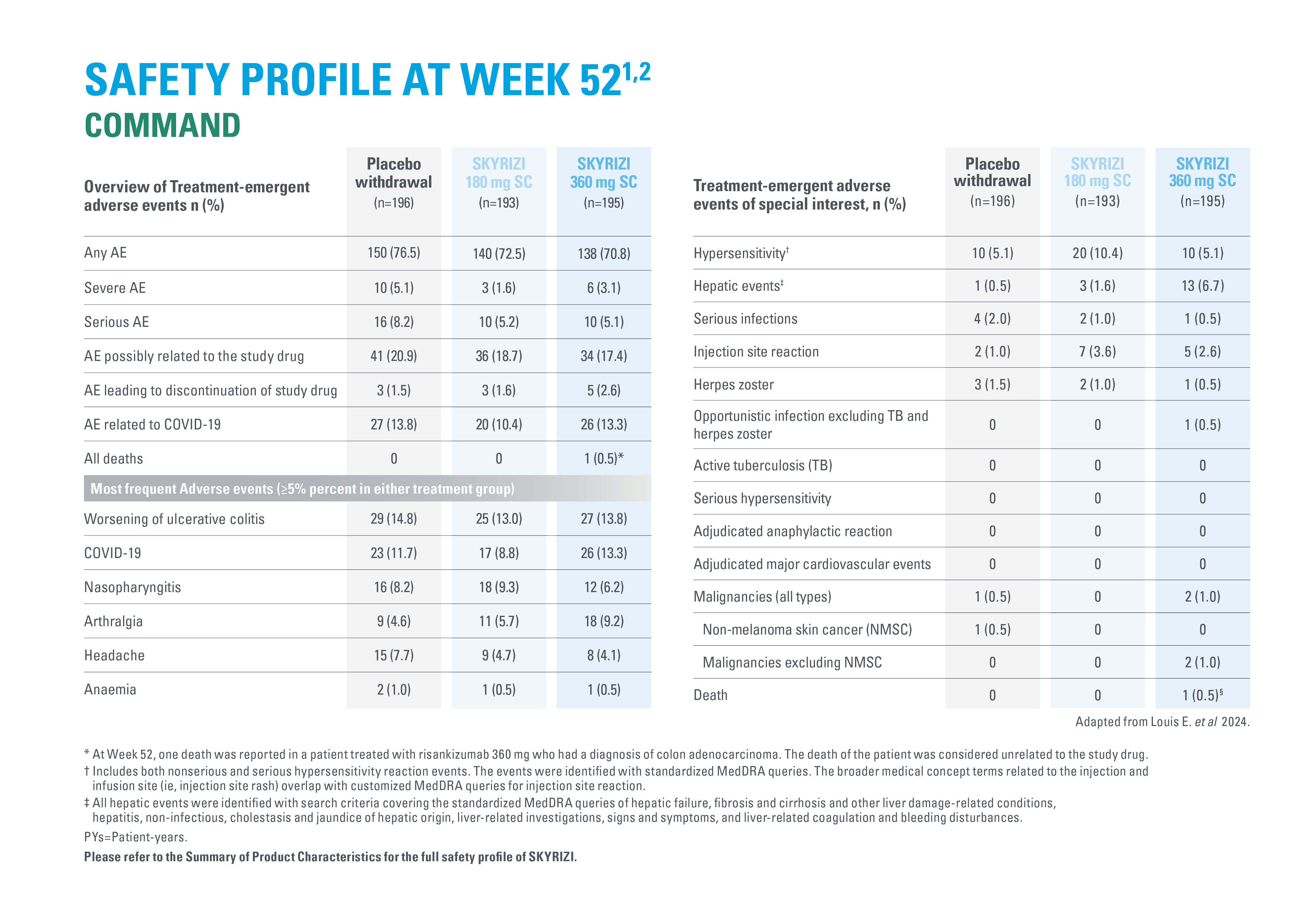
ADVERSE REACTIONS
For full safety information please refer to the Skyrizi® Summary of Product Characteristics available at www.medicines.ie.
References:
1. SKYRIZI Summary of Product Characteristics (SmPC) available at www.medicines.ie
2. Louis E, et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA. 2024;332(11):881–897.
For full safety information please refer to the Skyrizi® Summary of Product Characteristics available at www.medicines.ie.
References:
1. SKYRIZI Summary of Product Characteristics (SmPC) available at www.medicines.ie
2. Louis E, et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA. 2024;332(11):881–897.
IE-SKZG-250048 | Date of preparation: January 2026