References:
1. SKYRIZI® Summary of Product Characteristics. Available at www.medicines.ie.
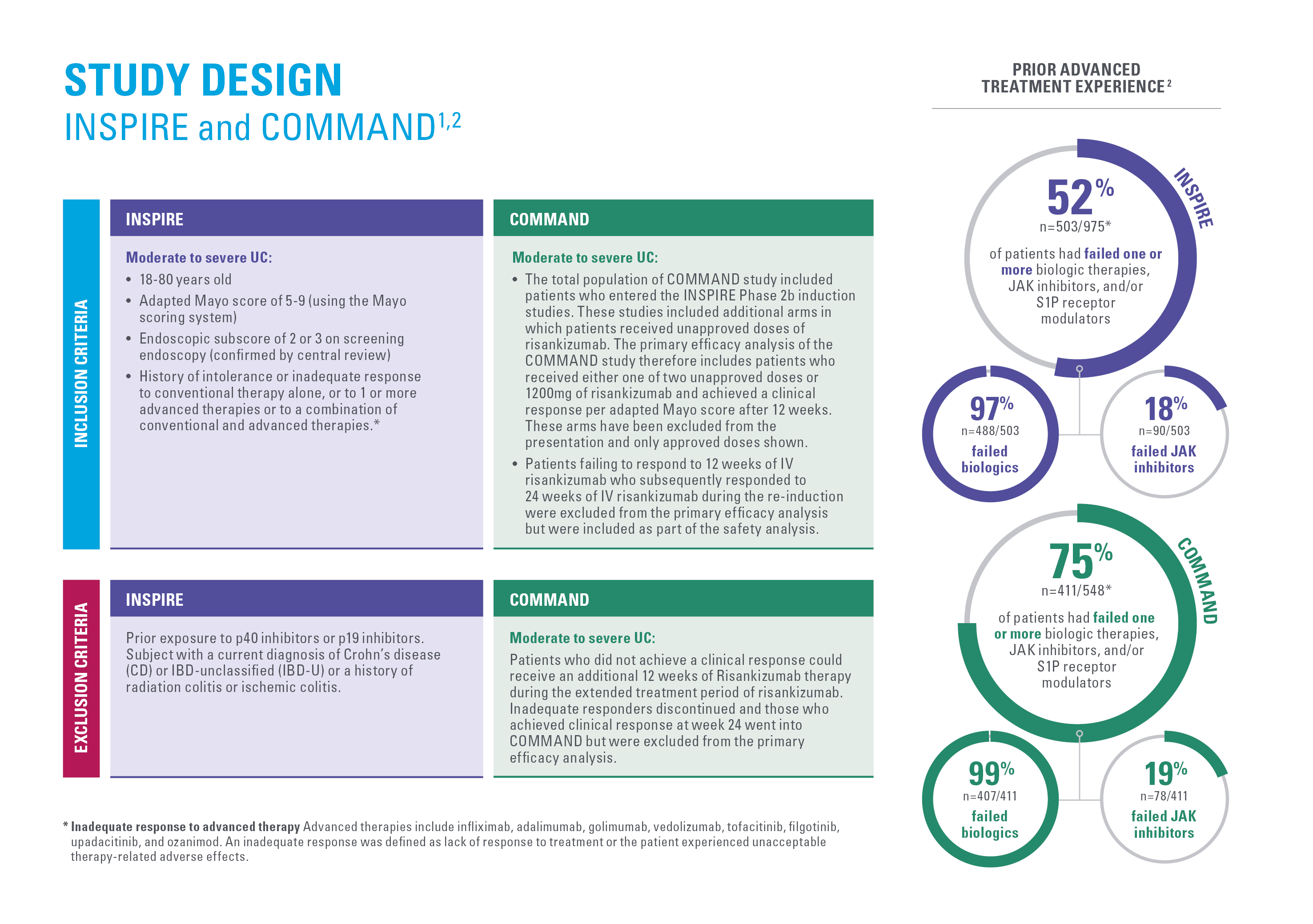
2. Louis E, et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA. 2024;332(11):881–897.
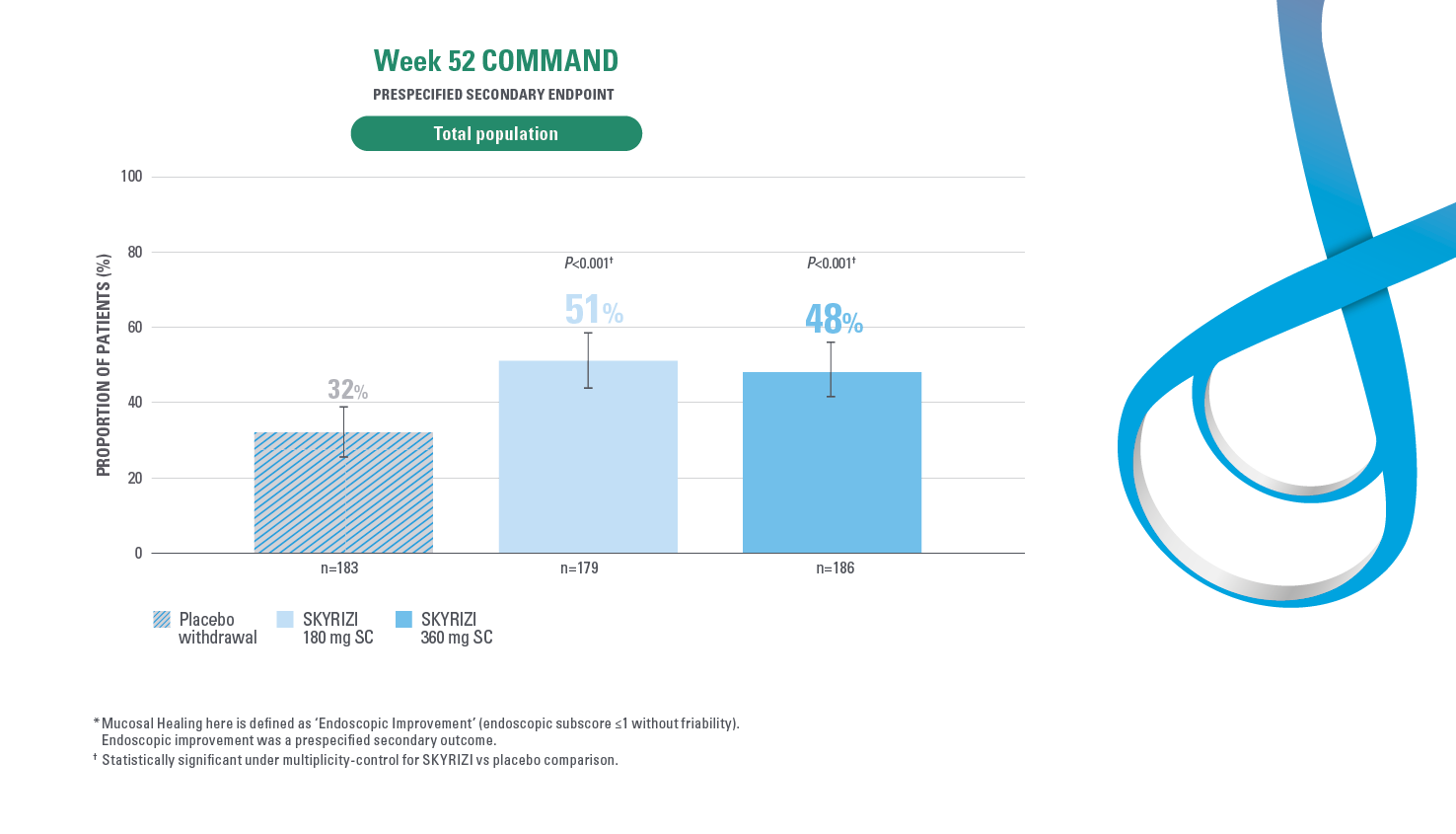
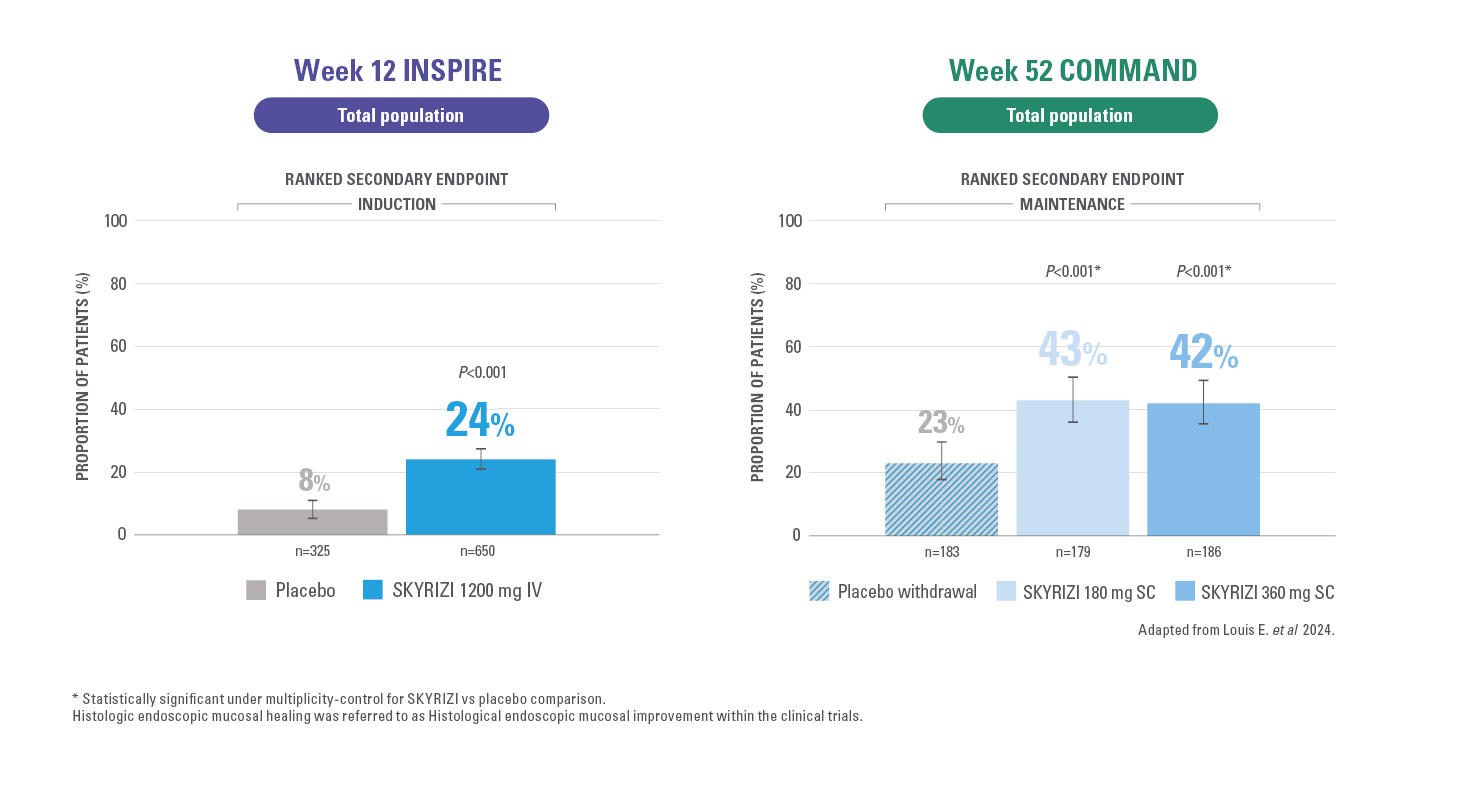
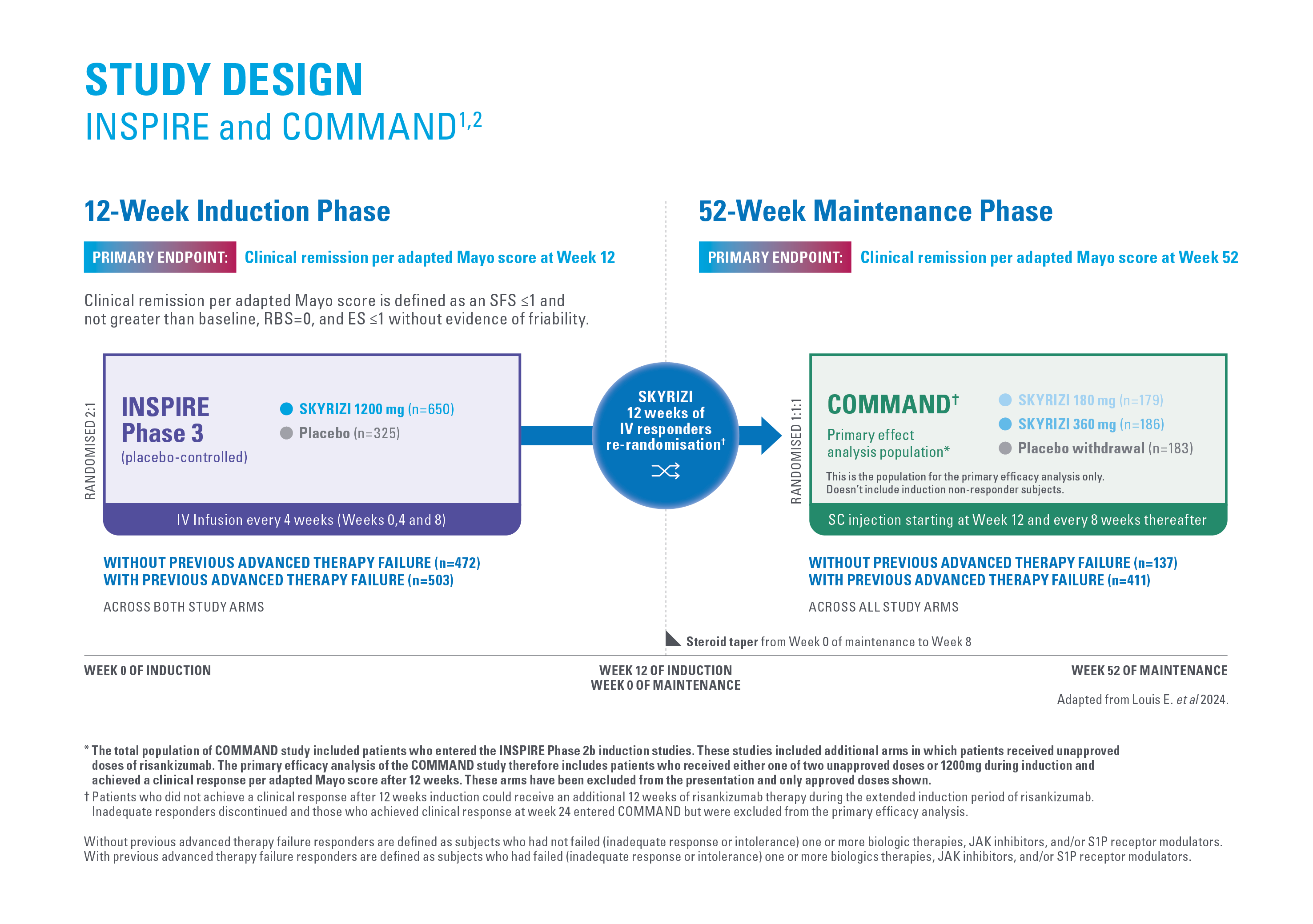
MUCOSAL HEALING* AT WEEK 521,2
Endoscopic Improvement (endoscopic subscore of 1 or less without friability) at week 52
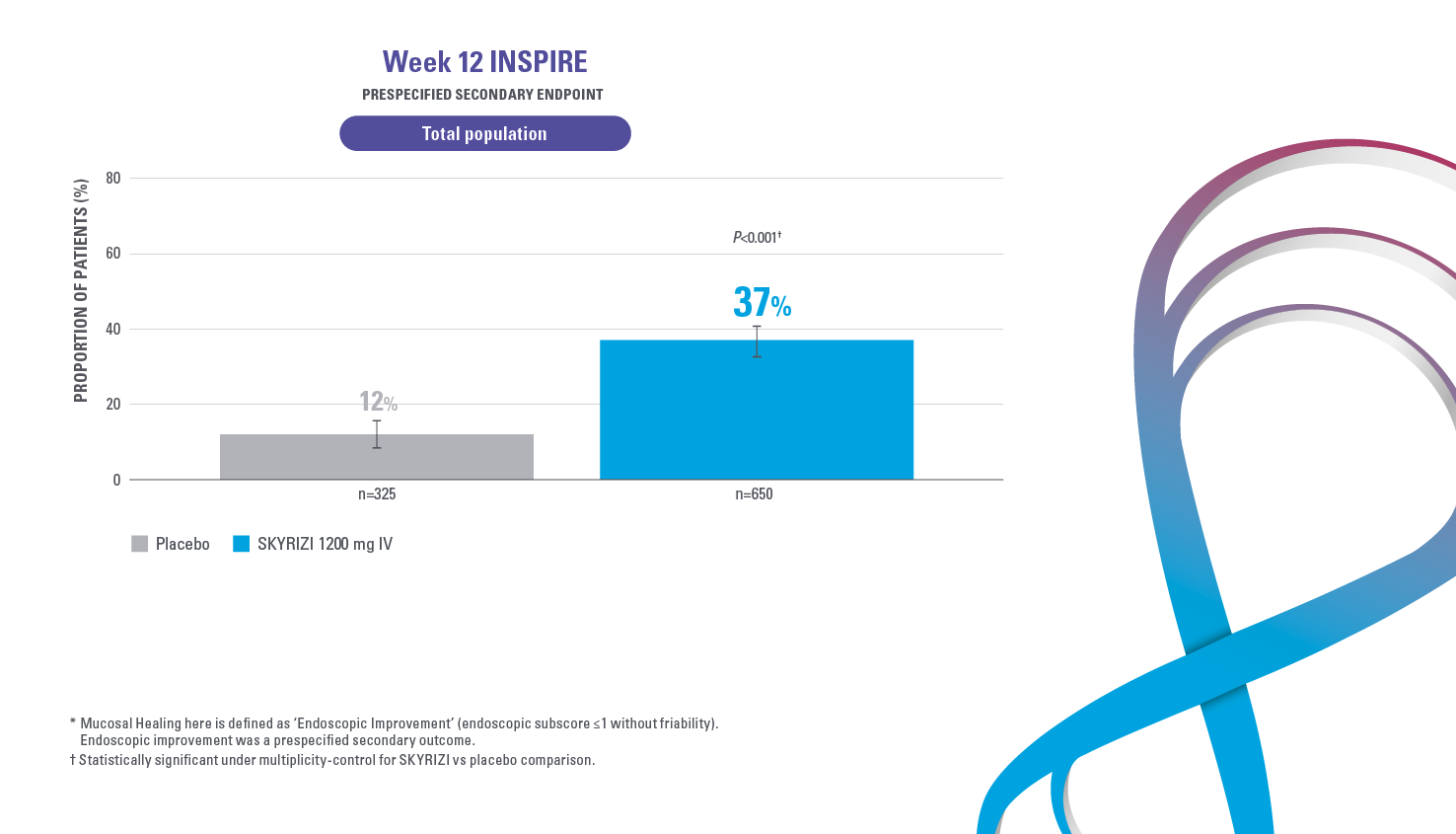
HISTOLOGIC ENDOSCOPIC MUCOSAL HEALING AT WEEK 121,2
Defined as an endoscopic subscore ≤1 without the evidence of friability and Geboes score ≤3.1.
References:
1. SKYRIZI® Summary of Product Characteristics. Available at www.medicines.ie.
2. Louis E, et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA. 2024;332(11):881–897.
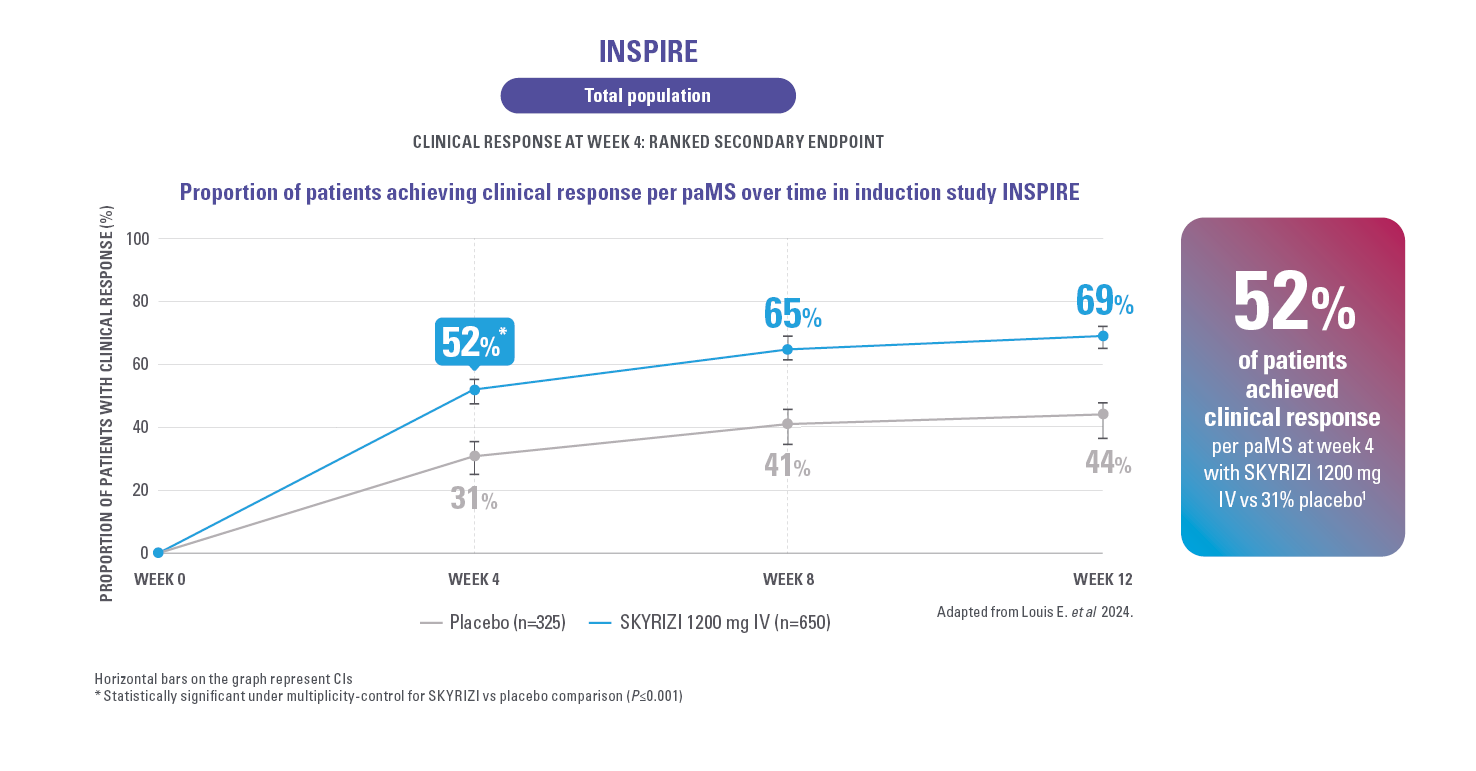
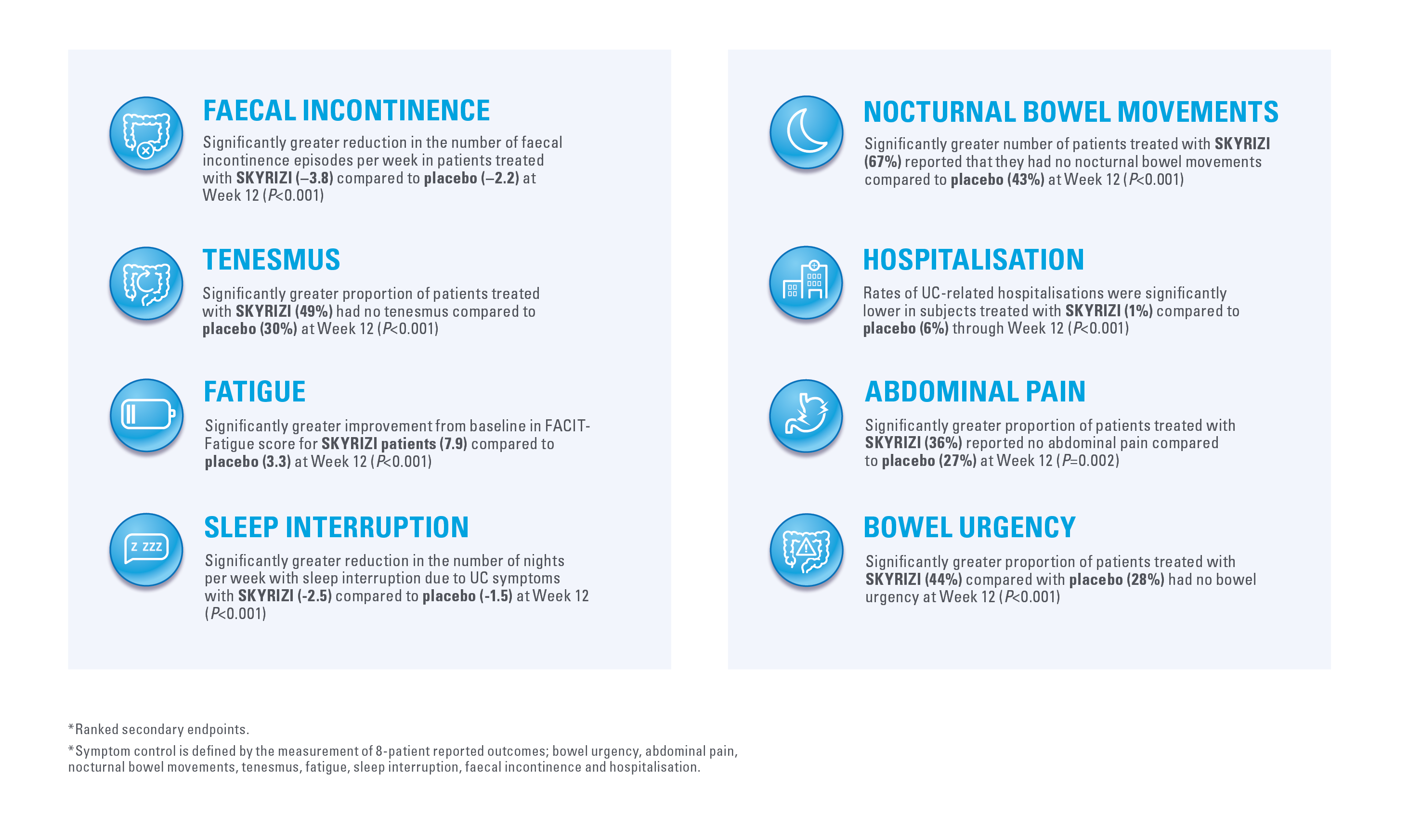
SYMPTOM CONTROL AND PATIENT OUTCOMES
AT WEEK 121,2*
References:
1. SKYRIZI® Summary of Product Characteristics. Available at www.medicines.ie.
2. Louis E, et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA. 2024;332(11):881–897.
IE-SKZG-250047 | Date of preparation: January 2026